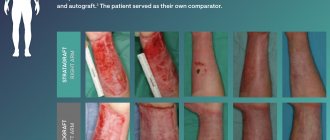
A burn is a common injury that can occur at home or at work. You can burn your skin with boiling liquid or steam, hot objects and particles of molten metal, aggressive caustic acids, alkalis and other chemicals. Electric shock and exposure to harmful ionizing radiation also cause damage to the skin. For small and shallow burns, conservative treatment is carried out. If the area of damage is extensive, and the damage affects deep skin structures, the only effective treatment method is skin transplantation after the burn.
Burn degrees
Based on the depth of tissue damage, burns are classified into 4 degrees.
- Damage to the surface layer (epidermis), accompanied by pain, redness and slight swelling of the burned area. Heals in a few days without special treatment.
- Damage to the epidermis and upper layer of the dermis, manifested by severe pain, swelling, redness, and the formation of blisters filled with clear serous fluid. With proper drug therapy, the injury heals in 10-14 days.
- A 3rd degree burn is classified into 2 types. Degree 3A is characterized by damage to the epidermis and dermis, the formation of large blisters with cloudy liquid inside, and a scab (crust) on the burned surface. Degree 3B is diagnosed when all skin layers are affected and subcutaneous fat is partially damaged. The resulting blisters are filled with bloody fluid.
- The most severe injury, accompanied by charring of the skin, muscle and bone tissue, and complete loss of sensitivity due to the destruction of nerve fibers.
Even a small but very deep burn is dangerous due to the penetration of infection and its spread through the bloodstream throughout the body. The results can be sepsis and death.
Kolkhuri
This anti-inflammatory drug is produced by Georgian pharmacological. It is an ointment with a pleasant herbal aroma. Colkhuri is developed on a plant basis and has a very wide spectrum of action. It is also used to treat burns.
Colkhuri ointment
Kolkhuri has the following properties:
- anti-inflammatory;
- antiseptic;
- painkillers;
- regenerative;
- immunostimulating.
Such a drug has no contraindications except for one thing – individual intolerance to the components included in its composition. It has proven itself in the treatment of joint problems, gout, myalgia and a number of other diseases.
Indications for transplantation
Skin grafting surgery is called dermoplasty (otherwise known as skin grafting, transplantation), and is prescribed in situations where it is impossible to restore burned tissue by other means.
Mandatory indications for dermoplasty are:
- 3B and 4 degree burns with a affected area of more than 2.5 cm;
- 2-3 degree burns with a large area of damage;
- formation of rough scar tissue, severe skin defects;
- trophic ulcers on the burned area.
2nd and 3A degree burns are borderline and are usually treated conservatively. Less commonly, to speed up regeneration and prevent complications, the doctor may recommend skin grafting.
For deep burns that occupy a large area, the operation cannot be performed until young granulation connective tissue has formed on the wound surface, so transplantation is carried out no earlier than 20-30 days after the injury. For small wounds with smooth edges and no areas of necrosis, plastic surgery can be performed within the first week after injury.
In children
Particularly severe burn injuries to the skin are diagnosed in children - according to statistics, more than half of childhood victims underwent dermoplasty. Skin grafting after a burn in children is mandatory, otherwise:
- the formation of rough scars, constrictions, noticeable skin defects causing functional impairment, physical discomfort and psychological disorders;
- improper formation of the musculoskeletal system (due to uneven stretching of healthy and scar tissue, tendons and muscle fibers become twisted).
Features of care and rehabilitation after transplantation
The recovery period can be divided into 3 periods. The first is 2-3 days after the procedure, when the skin adjusts to itself. The second stage is regeneration, which takes 2-2.5 months. During this period, the area with the transplanted skin should be protected from various types of damage. The bandage is removed only with the consent of the doctor.
Wound care is an important part of postoperative care. The procedure is performed only in the clinic using sterile materials. For treatment at home, the doctor prescribes painkillers and also uses special ointments to maintain water balance in the wound. The main thing is not to allow the skin to dry out at the transplant site, otherwise severe itching will be felt. The recommendations given by the doctor before discharge are as follows:
- Timely change of dressing;
- bed rest;
- Do not wet the wound;
- Drinking;
- giving up alcohol;
- taking vitamins;
- Proper nutrition.
The third stage of recovery is rehabilitation. It takes 3 months for complete recovery. If you follow all the doctor’s instructions, the recovery period passes quickly and without visible complications. After this, the person will be able to return to their normal lifestyle.
Contraindications
Contraindications to dermoplasty are:
- absence of young connective (granulation) tissue with extensive damage;
- areas of necrosis on the wound;
- inflammation in the affected and nearby healthy areas;
- discharge of serous or purulent exudate;
- multiple hemorrhages, accumulation of liquid or coagulated blood in soft tissues (hematoma).
Relative contraindications include the unsatisfactory physical condition of the patient:
- burn shock;
- loss of a large volume of blood;
- exhaustion;
- low hemoglobin and poor indicators of other laboratory tests.
Iruksol
The ointment is intended for the treatment of wounds of various origins, including I-III degree burns. Its manufacturer is Smith Nephew (Germany). The main active ingredients are chloramphenicol and clostridiopeptidase.
Iruksol ointment
The drug affects the affected area in several directions at once:
- cleanses the burn of contaminants;
- resolves dead cells, tissue, pus and scabs;
- destroys bacteria and pathogenic microorganisms;
- by stimulating the granulation process, it promotes faster healing of wounds.
According to doctors and patients, Iruksol is a very good ointment after burns, since it has a complex effect, but at the same time selectively (does not damage living tissue). The drug stimulates blood microcirculation without causing bleeding. It accelerates the regeneration of the skin and has the ability to resolve scars.
Preparation for dermoplasty
Primary dermoplasty is performed 3-4 days after the burn with a small wound area and does not require preparatory measures.
But more often, secondary (20-30 days after injury) skin grafting is performed after a burn. In this case, preparation of the wound for surgical intervention is mandatory: mechanical cleansing and drug therapy aimed at removing purulent and necrotic contents, preventing or treating infectious complications that have already arisen. Measures are also taken to stabilize and improve the patient’s general physical condition.
The preparation stage includes:
- prescription of vitamins and restoratives (to increase the body's resistance);
- systemic antibacterial therapy;
- the use of local antiseptics and antibacterial drugs (the application of ointments under the bandage is stopped 3-4 days before the planned date of transplantation, since remaining particles of drugs may complicate the engraftment of the graft);
- antiseptic baths with a solution of potassium permanganate or furatsilin;
- UV irradiation of the wound surface;
- blood transfusion (blood transfusion) or plasma transfusion (carried out according to indications).
The results of recent research in the field of biochemistry, pathophysiology, and immunology have explained some previously unknown molecular cellular mechanisms of chronicity of wounds of various etiologies, including those accompanying a number of common and rare dermatoses, and the stimulus in the search for new, more effective methods of treating them is still does not weaken. Treatment methods that have been proposed relatively recently include cellular technologies and the creation of skin equivalents.
The first attempts to use cellular technologies in the treatment of wounds of various etiologies, as well as burns, were made in the middle of the 20th century. Since then, many different cell technologies have been proposed, including: in vitro
skin cells (keratinocytes, fibroblasts), application of a suspension consisting of autocells to the wound, tissue-engineered structures (“living dermal equivalent”, “living skin equivalent”), as well as biosynthetic coatings that imitate skin [1, 2].
For the first time, it is possible in principle to grow skin cells, in particular keratinocytes, in vitro
, was shown in 1948 in the work of P. Medawar [3, 4], which marked the beginning of a new direction in the creation of biological coatings.
In 1977, G. Green et al. [5, 6] developed a method for culturing skin cells and obtaining large-area epithelial layers, which made it possible to use them in practice.
The first successful transplantation of autologous keratinocytes from a primary culture onto rabbit wounds was carried out in 1971 by M. Karasek [7, 8]. Subsequently, various substrates from biological tissues (in particular, pig skin) were used to obtain keratinocytes, which made it possible to improve the cell yield at each passage [9, 10].
In 1975, Rheinwald and H. Green [11, 12] developed a technology for serial cultivation of large quantities of human keratinocytes and successfully applied it in combustiology.
It has now been proven that transplantation of human skin keratinocytes promotes the closure of burn wounds and wounds of other origins of various sizes by the epidermis [13-15]. However, culturing autokeratinocytes takes a long period of time (3-4 weeks). During this time, the wound becomes infected and cell engraftment does not occur. In addition, the use of autokeratinocytes makes it impossible to create a bank of cell transplants: a mandatory element of obtaining significant volumes of keratinocytes is the use of numerous expensive biological stimulators of epidermal cell growth, which significantly increases the cost of the resulting material and limits its shelf life [2, 16].
In the 90s of the twentieth century at the Institute of Surgery named after. A.V. Vishnevsky RAMS D.S. Sarkisov et al. [17, 18] an original and effective method for treating burn wounds was developed based on the use of cultured cells, which was fundamentally different in that not keratinocytes, but fibroblasts were used for the first time as the main component of the cultured layer of cells. Fibroblasts are a heterogeneous population of mesenchymal cells and play a key role in the processes of regulating cellular interactions and maintaining skin homeostasis. Fibroblasts not only create optimal conditions for the functioning and proliferation of other types of cells (epithelial, endothelial, hair follicle cells), but are also responsible for coordinating their functions in accordance with their location on the body. Research results by D.S. Sarkisova et al. [18, 19] predetermined the possibility of using fibroblasts as the main element of a cell transplant in the treatment of wounds of various etiologies. The ability of fibroblasts to form the intercellular matrix, synthesize cytokines, and induce migration and proliferation of different types of cells during skin damage has made them promising for wide clinical use [20].
The research results obtained to date have shown that the use of cultured allofibroblasts reduces the need for donor skin resources, ensures rapid epithelization of the wound surface and timely restoration of the integrity of the skin [17, 21-23]. However, this method has a number of disadvantages. It takes 3-4 days to grow allofibroblasts; transplantation of allocells can provoke sensitization of the body by foreign antigens; It cannot be guaranteed that the donor will not have severe infectious diseases. It should also be taken into account that shortcomings in the financing of the healthcare sector, as well as in legal regulation in Russia, at one time limited the possibility of introducing this technology into clinical practice [2].
In the early 50s of the 20th century, R. Billingham and J. Reynolds [24, 25] made an attempt to use autologous epidermal cells for the treatment of wounds. In this case, keratinocytes were not cultivated, but only the epidermis was separated from the dermis using the enzyme trypsin and a cell suspension of epidermocytes was obtained, which was transferred to the prepared wound bed. This kind of transplantation led to the appearance of islands of epithelialization in the wound, which then merged with each other.
In 1993, F. Wood et al. [26] developed and patented the so-called spray on skin
— a method for obtaining autokeratinocytes and their transplantation in the form of a suspension and spraying onto wound surfaces, including large areas. Later in the UK, taking into account the clinical development of F. Wood by Avita Medical Ltd. [27] created ReCell, a technology that makes it possible to obtain a cell suspension consisting of autologous keratinocytes and melanocytes in a short period of time (20–30 min). To prepare the cell suspension, a “whale” was needed to collect autologous cells. A biopsy sample with a thickness of 0.2-0.3 mm, measuring 1×1 or 2×2 cm, depending on the area of the lesion, was obtained from the donor skin area using a dermatome. A solution of the trypsin enzyme was prepared, which was injected into a glass vial of a working device for heating. The biopsy material was placed in a trypsin solution for 15-20 minutes. Then the epidermal layer was separated from the dermal layer. Autocells were scraped from the surface of the dermal layer with a scalpel. To prepare the suspension, cells were diluted with sodium lactate. The resulting cell suspension was purified in a cell filter. A filtered suspension of autocells was applied to the wound by drop or spray.
Another direction in skin tissue engineering is the use of autofibroblasts [28]. To create 1.5 cm2 of autologous tissue, a skin biopsy with an area of 3 mm2 is required, while the time for cultivating the smallest amount of donor material is on average 3 weeks [29, 30]. At the first stage of cultivation, a primary culture of fibroblasts is obtained by fragmentation and enzymatic treatment of the dermis with a trypsin solution. Cell cultivation is carried out in Eagle nutrient medium (DMEM) with the addition of 10% fetal calf serum. Next, the primary culture is passaged, subjected to 4-7-fold subcultivation [30]. Subcultivation is carried out on a membrane, with the help of which the culture is transplanted into the wound. Control of culture contamination with bacteria, mycoplasmas and viruses is carried out by microbiological and cytogenetic methods at the first passages and when creating cell culture banks. Certification of cell cultures for karyotype stability and tumorigenicity is carried out in accordance with WHO requirements [30, 31].
A new stage in the development of bioengineering technologies in the treatment of long-term non-healing wounds was the creation and use of a “living equivalent of skin”, which is a collagen gel containing microcarriers of allo- or autofibroblasts and covered with a culture of allokeratinocytes [32-36]. The "living equivalent of dermis" is similar to tissue. Its basis is a collagen gel populated with fibroblasts, and keratinocytes are grown on the surface of the gel [37]. The creation of this material is a labor-intensive process performed in special laboratories; it requires the development of technology for collecting and culturing cells, creating a bank of cell cultures, and introducing a quality control system.
One of the modern trends in creating living skin equivalents is the use for culturing stem cells, in particular mesenchymal cells (MSCs), as well as human amniotic epithelial cells (hAECs), which, together with fibroblasts, are able to recreate in vitro
structure of the skin and basement membrane [38–41]. Research is being conducted to enhance regenerative capabilities and enhance vascularization in commercially available skin equivalents through the use of activated human skin-derived stem cells (SDSCs) [42]. Three-dimensional (3D) printing (3D bioprinting) has been used to create artificial skin, which is an important component of regenerative medicine designed to meet the need for tissues and organs suitable for transplantation [43].
One of the important issues in cell medicine is the transfer of in vitro
cells into the human body. Since cell cultures are substrate dependent and grow attached to the culture plastic, there is a need for enzymatic treatment of cells and their transfer into suspension before transplantation, as discussed above (ReCell technology). As a result of such manipulations, cell viability is significantly reduced, which certainly affects the quality of transplantation. The use of various bioplastic materials as substrates made it possible to avoid these problems, since, compared to suspension cell transplants, they increase cell survival and ensure their more active proliferation due to adhesion to the matrix; act as a volume-forming agent and promote active induction of angiogenesis and reparative regeneration [44].
Various materials based on porcine dermis [45], amniotic membrane [46], bladder submucosa [47], synthetic biodegradable and non-biodegradable materials are currently used as a structural basis for tissue-engineered structures, including living tissue equivalents of skin. [48, 49], as well as collagen gel, which was one of the first to be used [37].
In order to optimize the structure and functions of substrates, work is currently being carried out on the design of two-dimensional (2D) and 3D matrices for tissue engineering structures, including 3-layer “sandwich” collagen scaffolds with different pore sizes that imitate the natural structures of human skin [50 ]. Research is being conducted to examine the effects of various biomaterials used as scaffolds (e.g. chitosan, gelatin, fibrin, bovine collagen, porcine dermis, collagen-glucosamignoglycan, nanomaterials) on key cell culture parameters (seeding efficiency, cellular distribution, survival, metabolic activity) [51-55].
Types of artificial leather
Currently, there are many different classifications of skin substitutes (Jones et al., 2002; Atiyeh et al., 2005; Horch et al., 2005; Atiyeh & Costagliola, 2007; Clark et al., 2007; McNeil, 2007; Patel & Fisher, 2008) [56]. Detailed coverage of the classification, as well as practical and theoretical aspects of the creation and application of tissue-engineered skin constructs and the prospects for using artificial skin in clinical practice are contained in reviews [2, 20, 56, 57].
Based on the type of biomaterial, skin substitutes are divided into biological (including autologous, allogeneic, xenogeneic) and synthetic (biodegradable and non-biodegradable).
Autologous skin is skin obtained from the patient himself (as a rule, we are talking about a patient with a burn of varying severity), followed by temporary closure of the wound surfaces. Autologous skin is obtained by repeatedly cutting skin from the same donor sites of the patient and from areas of the body where epithelization of superficial burn wounds has completed. A particularly valuable donor site for cutting split skin flaps is the scalp. Due to the large number of skin appendages (hair follicles and sebaceous glands) and good blood supply, epithelization of wounds occurs quickly. Split skin from the scalp can be cut off repeatedly (up to 7 times or more) [58]. Due to the insufficient resources of their own skin, it is not possible to quickly restore the skin of those seriously burned using traditional methods of autodermoplasty. For this reason, improving methods of economical skin grafting remains relevant.
Allogeneic human skin (cadaveric or living donor) is the gold standard for wound dressing [59]. When transplanted, physiological occlusion of wounds is achieved; it prevents the penetration of microorganisms into wounds and promotes epithelization of donor wounds and IIIa degree burns. When using allogeneic tissues, the need for autologous skin during surgical restoration of the skin is reduced. As a rule, skin is obtained from corpses within a short time (no more than 6 hours) after death. For wound closure and combined plastic surgery, cadaveric skin can be used, the preservation and storage of which was carried out in various ways. As a rule, the preservation procedure worsens the biological properties of the leather, so it is better to use so-called fresh leather with a short shelf life. At the same time, allogeneic skin is a powerful allergen and causes severe sensitization of the body. This sometimes leads to the melting of previously engrafted flaps and autoskin, including against the background of immunosuppressive therapy.
Xenoskin is a medical skin substitute. Xenoskin is most often obtained from pig skin. It is used in medicine mainly in the treatment of burn patients. When producing xenoskin, a layer of pig skin 0.3-0.4 mm thick is cut off, then preserved in nitrogen, dried and packaged in sterile bags of 100, 200 or 300 cm². Leather in such a bag can be stored for up to 3 years. Before use, xenoskin is soaked in saline or antiseptic, applied to the wound and secured with special bandages. In most cases, when this material is used, after 1-2 weeks the xenoskin on the wound dries out and falls off. Instead, normal human skin is formed. The advantage of xenoskin is that suppuration rarely occurs under it, which improves the healing of burns and other extensive wounds. The cost of this product is in most cases very reasonable, which makes it accessible in developing countries. The use of xenoskin is advisable when more than 50% of the victim’s skin is affected. However, animal skin causes severe immune reactions in some people. The α-1,3-galactose (Gal) fragment present on the membranes of pig cells, to which antibodies are present in human blood, is responsible for rejection. In order to solve this problem, recently studies have been conducted on the possibility of using the skin of low-immunogenic pigs, in which the Gal epitope is not expressed, due to the selective knockout of the gene encoding α-1,3-galactosyltransferase. The use of grafts from the skin of low-immunogenic pigs for temporary healing of burn wounds in primates has significantly increased the viability of the graft compared to the skin of conventional pigs and with an allogeneic graft [60, 61].
At the moment, among biological coatings, with legislative restrictions on the use of cadaver skin, the most common are preparations of pig dermis (Alloask D, Xiderm, Xenoderm, Swiderm, etc.). In the table
Commercially Available Products Prepared for Surgical Use Commercially available products prepared for surgical use are presented.
Synthetic wound coverings applied to wounds after scab removal are themselves an important element of treatment. They not only prevent exhaustion of the body and infection of wounds, but also prepare wounds for subsequent skin grafting. Wound dressings used after removal of the scab must be highly adhesive and quickly attach to the surface of the wound, be impervious to water and have limited permeability to vapor and water, reduce heat loss through wound surfaces, reduce the loss of proteins and electrolytes, prevent microbial invasion of wounds, reduce feeling of pain, facilitate physiotherapeutic measures, ensure painless dressings, do not undergo proteolytic digestion, help cleanse wounds from tissue detritus, reduce the healing time of deep dermal and donor wounds, have a hemostatic effect, improve the general condition of the patient. Synthetic wound coverings have a number of important advantages over tissues of natural origin: they do not lose their properties during storage, do not require replacement on wounds, and are convenient to use. Among the large list of such drugs are Foliderm, Biobran, Sispusderm, Omiderm, Epigard, Syncrite, or Sincover. A particular advantage of these coatings is the ability to constantly have sterile and relatively cheap material in unlimited quantities. However, imported drugs are often unavailable due to their high cost.
According to the anatomical structure, skin equivalents (substitutes) are divided into epidermal, dermal and dermoepidermal [20, 56, 62].
Epidermal skin substitutes have been developed since it became possible to serially culture them in vitro.
human keratinocytes.
These technologies quickly found their clinical application, which contributed to improving patient survival rates. However, the significance of cultured keratinocytes remains controversial to this day. The key to the production of an epidermal substitute is the isolation of keratinocytes from a donor and their subsequent in vitro
to obtain the required number of keratinocytes for therapeutic needs. There are differences in approaches to the production of epidermal substitutes, which depend on culture methods, stage of cell differentiation and epithelial organization (confluent sheets, subconfluent cell sheets and suspensions); methods of delivering cells to the patient (in the form of confluent sheets, aerosol or using microcarriers), as well as the use of additional supports to increase the efficiency of cell culture delivery.
In order to obtain a culture of autologous cells, a skin biopsy measuring 2-5 cm2 is performed upon the patient's arrival at the clinic. The epidermis is separated from the dermis and the keratinocytes are released by enzyme action. The resulting keratinocytes are seeded into a culture vessel where single cells begin to divide to form colonies in the presence of mitotically inactivated mouse fibroblasts and a culture medium containing fetal calf serum and necessary supplements. Single colonies of keratinocytes fuse together and form epithelial layers, which can be enzymatically detached from the culture flask and placed on backing media (such as paraffin, gauze) to maintain the basal-apical orientation of the cells, and then applied to the wound. The quality of such stratified cultured epithelial autograft (CEAS) depends on the clonal cellular composition, which determines graft survival and longevity when used in vivo. Other disadvantages of CEAS include long culture times, fragility of grafts, and complex processing and procedural complexity. There is also a need for precise timing between culture acquisition and delivery to the clinic. The main disadvantage of using the leaf is unpredictable clinical outcomes.
Commercially available epidermal skin substitutes include Epicel (EPIBASE, EpiDex), MySkin, Laserskin (Vivoderm), Bioseed-S, CellSpray [56].
Dermal skin substitutes.
The main structural components of the dermis are cellular and fibrous elements, as well as the interstitial substance. Fibrous elements are mainly represented by collagen and elastin fibers, the interstitial substance is glycoproteins, proteoglycans and glycosaminoglycans. The main functional cellular element of the dermis is the fibroblast. The cell population of fibroblasts is the source of the formation of almost all structural components of the dermis, therefore, when creating skin substitutes, most scientists use a collagen substrate mixed with fibroblasts and glycosaminoglycans, which serves as the main component of the dermal equivalent [63-65]. The main advantage of the dermal equivalent is due to the fact that the cells in it are in an active functional state, close to those in the skin [66-68]. However, the conventional dermal equivalent is not a durable construct, as the fibroblasts included in the gel cause it to rapidly contract. In this regard, the biological structure cannot be stored for a long time, which makes its use inconvenient.
Most commercially available dermal skin substitutes are acellular and are based on allogeneic, xenogeneic or synthetic materials. Obtaining a license for their production is much easier compared to structures containing cells and double-layer skin substitutes. The ability to produce large batches of products has led to their fairly wide distribution and use in the clinic. Commercially available products include AlloDerm, Karoderm, SureDerm, GraftJacket containing acellular human dermal matrix products, as well as products such as OASIS Wound Matrix, EZ Derm, Integra Dermal Regeneration Template, Terudermis, Pelnac Standard Type/Pelnac, Fortified With Mesh Type, Biobrane, Biobrane-L, TransCyte, Dermagraft Hyalomatrix PA, Hyalograft 3D [56].
The dermal equivalent is not full-fledged skin, since its structure lacks the surface layer of skin - the epidermis. To create full-thickness skin and more quickly restore the viability of the transplanted skin equivalent, a layer of keratinocytes is applied to the surface of the dermal matrix. This tissue product is called a living skin equivalent [69, 70] and is a dermoepidermal substitute.
Dermoepidermal substitutes mimic the histological structure of normal skin, where both epidermal and dermal layers are present. There are also some functional similarities to normal skin. These are not only the most advanced and sophisticated technologies and products compared to epidermal and dermal substitutes, but also the most expensive biological constructs in skin tissue engineering.
One of the first options for a living skin equivalent was proposed by E. Bell in 1983 [37]. The dermal component was represented by a gel consisting of skin fibroblasts, collagen, plasma and growth medium. Keratinocytes were grown on the surface of this gel over a period of 1 to 2 weeks. Transplantation of allogeneic keratinocytes created conditions for marginal epithelialization due to the synthesis of basement membrane components, associated cytokines and growth factors [71]. As a result, a 3D model of the skin was created, representing viable tissue in the form of a translucent elastic mass.
Cellular structures in the form of living skin equivalents are used both for deep burns and for other deep three-dimensional defects of the skin. In addition, they are an excellent model for studying the processes of regeneration and morphogenesis in vitro
[2, 72].
The undoubted advantage of the living equivalent of skin is that as a result of its engraftment, morphofunctionally complete skin is formed.
Most existing commercially available dermoepidermal skin substitutes are based on the use of allogeneic skin, which allows for the production of large quantities of a uniform batch of product with relative affordability. Nevertheless, these biomaterials act more like temporary biologically active dressings that provide the skin of a sick person with growth factors and cytokines during the healing process of wounds. The most famous of them are Alligraft, Karoskin, Apligraft, Or-Cel, PolyActive [56].
2D and 3D artificial leather models
Skin equivalents known to date are most often cultured on 2D substrates, such as culture plates, or in 3D matrix conditions. It has been shown that cells cultured on 2D substrates grow in conditions that do not correspond to natural environmental conditions, lose many important intracellular signals, key regulators and tissue phenotypes, and are unable to reproduce the complex dynamic processes occurring in the skin under natural conditions [ 73].
Cells growing under 3D conditions have receptor expression on the cell surface, pronounced proliferative capacity, and metabolic functions [73–75]. It has been shown that only under 3D conditions do keratinocytes form an ordered epithelium [74–78]. Thus, strategies that allow the construction of artificial equivalents of human skin in 3D conditions, including dermal and epidermal components, allow the study of cell physiology, which cannot be accomplished in a tissue culture monolayer.
Applied aspects of artificial leather
Technologies for creating microtissues can be used to treat defects of the integumentary epithelium, recovery after operations, injuries, burns, fistulas, and in reconstructive surgery. Currently, it is known to use methods of transplantation of allogeneic cultured cells (fibroblasts and keratinocytes) in combustiology in the treatment of long-term non-healing granulating skin wounds, trophic ulcers and fistulas, to replenish the vocal cords and soft tissues of the face with fibroblasts [79-82].
Skin equivalents are currently being studied and used clinically for a number of skin pathologies. According to the literature [83–100], diseases for which human skin equivalents can be used include psoriasis, vitiligo, keloids, nevi, genodermatoses such as xeroderma pigmentosum and congenital epidermolysis bullosa.
3D skin models can be successfully used in scientific research [101]. These models make it possible to study the basic architecture of human skin, intercellular interactions, the influence of the environment on the regulation of melanogenesis, proliferation and differentiation of keratinocytes, as well as patterns of epithelialization processes in wounds. These skin models also provide a platform for modeling photoaging and skin cancer, and are an excellent system for conducting pharmacological studies to test drugs and evaluate their pharmacological activity and toxicity. Such studies are carried out on microtissue samples simulating normal tissue, diseases, and tumors of various origins. In addition, 3D equivalents of human skin can be constructed with specific genetic changes in the dermal or epidermal compartments of the model and serve as a basis for studying the influence of the environment on these modifications. 3D skin models are also a cost-effective alternative to the use of laboratory animals, in particular in toxicological studies, in assessing the safety of cosmetics, phototoxicity and genotoxicity of various reagents [102–104].
Human skin substitutes (equivalents) are currently commercially available, but there are a number of challenges regarding preclinical safety assessment. In particular, when providing a skin equivalent for clinical use, companies must demonstrate that it is pathogen-free, immunogenic, tumorigenic, and has normal physiological functions.
The authors declare no conflict of interest.
1e-mail 2e-mail
Operation technique
OLYMPUS DIGITAL CAMERA
Skin grafting for burns is carried out in several stages.
- A special instrument is used to collect material for transplantation. This stage is omitted if the graft material is not your own skin.
- The burn wound is prepared for transplantation: the wound surface is cleaned, necrotic tissue is removed, rough scars along the edges of the wound are excised, the wound bed is leveled, treated with an antiseptic, and irrigated with an antibiotic solution.
- The graft is aligned with the edges of the wound surface and, if necessary, fixed with catgut (self-absorbable suture material), a mixture of fibrin and penicillin solutions.
- The donor material is irrigated with glucocorticosteroid solutions to reduce the risk of rejection.
- Moist cotton balls and swabs are applied to the transplanted material, and a pressure bandage is placed on top.
Materials used
There are various synthetic and natural materials used for dermoplasty:
- autoskin – own skin taken from an unburned area of the body that is usually hidden by clothing (inner thigh, back, buttocks);
- allo-skin – cadaveric skin (taken from a dead person), preserved for subsequent use in transplant surgery;
- xenoskin – animal skin (usually pig skin);
- amnion – the embryonic membrane of the embryo of humans and higher vertebrates (reptiles, birds, mammals);
- artificially grown collagen and epidermal materials.
Skin grafting is most often performed by specialists using biological materials – autoskin and allo-skin. Amnion, xenoskin, and other synthetic and natural materials generally cover the wound surface temporarily in order to prevent infection.
When choosing the appropriate material, the depth of the burn is also taken into account. For grade 3B and grade 4 injuries, it is recommended to use your own skin, while for grade 3A injuries, plastic surgery is performed using allo-skin.
When transplanting your own skin, flaps taken from a certain area of the body can be used, which are not connected in any way to other tissues and organs. This type of surgery is called free plastic surgery. Surgery using the skin around the wound, which is moved and stretched over the burn area using microscopic incisions, is called non-free plastic surgery.
The quality and physical characteristics of the graft depend on the thickness of the flap. According to this parameter, donor materials are classified into 4 types.
- Thin flap (no more than 20-30 microns). Includes the epidermis and the basal layer of the dermis. It is characterized by poor elasticity, often wrinkles, and is easily damaged, therefore it is used very rarely for burns, mainly for temporary covering of the burn area.
- A flap of medium thickness, otherwise intermediate, split (from 30 to 75 microns). Consists of epidermis and dermis (full or partial layer). It is characterized by high strength and elasticity, practically no different from your own healthy skin. It is most often used for transplantation after burns; it is suitable for restoring skin on moving areas (articular surfaces), since it has sufficient elasticity and does not impede movement.
- Thick flap (50-120 microns). Includes all layers of the skin. Used for very deep wounds or damage to open areas of the body (face, neck, décolleté). It is transplanted only to areas where there is a sufficient number of capillaries that will connect to the vessels of the donor flap.
- Composite flap. In addition to the skin itself, it includes subcutaneous fatty tissue and cartilage tissue. Used for facial burns.
About the recipient site
After surgery, the recipient site may be covered with a pressure or vacuum bandage.
Pressure bandage
A pressure bandage will help the recipient site heal properly. It exerts pressure on the recipient area, due to which liquid does not accumulate under it. This allows the skin flap to adhere to the skin. It can be secured with silk sutures, a splint, a plaster cast or a support bandage. This will prevent the flap from moving.
Your surgeon or nurse will remove the pressure bandage approximately 5 to 7 days after surgery. Once the pressure dressing is removed, the recipient site will be covered with a Xeroform dressing.
If you have a cast, your surgeon will cut out a portion of it above the recipient site. This will allow him to examine the flap. The cast will be removed 10 days after surgery unless you have had other surgeries. If you have had other surgeries, you may need to wear the cast longer. An Ace® bandage, gauze bandage, or tape will be used to secure the Xeroform cast after the cast is removed.
You may be told to change the Xeroform dressing and gauze once a day until the flap is completely healed. Your nurse will teach you and your caregiver how to change the dressing.
Vacuum wound dressing
Instead of a pressure bandage, the surgeon may use a vacuum bandage on the recipient site. A vacuum dressing is a special dressing that suctions fluid from a wound, which speeds up healing.
The vacuum wound dressing will be removed 5 to 7 days after surgery. The surgeon will then examine the flap to ensure it has completely healed. If the flap has not healed completely, you may need to wear the vacuum dressing longer.
to come back to the beginning
Complications
One of the main complications of skin grafting is graft rejection, which can occur even after transplanting your own skin. The reason for this phenomenon is most often the presence of remains of pus, necrotic cells, and medicinal substances in the wound.
When rejected, complete or partial necrosis of the transplanted skin occurs. In this case, the dead tissue is removed, after which a re-transplantation is performed. If the rejection is partial, only the foci of necrosis are removed, leaving the engrafted tissue.
Other common complications of skin grafting include:
- bleeding of postoperative sutures;
- tissue compaction and scar formation along the edge of the wound surface (at the junction of healthy and donor skin);
- secondary infection of the wound (if the rules of preoperative preparation are violated or asepsis is not observed during surgery);
- sepsis;
- cracking of the donor skin, the appearance of ulcers and erosions on its surface, limitation of movements due to tightening, especially on the articular surfaces (occurs when the wrong material is chosen or the transplantation is not carried out in a timely manner);
- decreased sensitivity, atrophy (decrease in volume) of tissue in the transplant area.
Microsurgical techniques for skin grafting for trophic ulcers
It is used when closing a wound over an open tendon or joint, while restoring skin integrity over supporting areas. The purpose of the technique is to form a flap of skin on a vascular base, which can be rotated in different directions without disturbing the trophism. The described method requires the hands of a professional, but if done, the result is excellent. There is a concept with preliminary isolation of a musculocutaneous flap with a vascular base and transplanting it to the problem area, where the vessels that will nourish it should also be “included” in the work.
Pros and cons of skin plastic surgery
Skin transplantation after a burn, like all other types of plastic surgery, has its advantages and disadvantages. The first include:
- protection of the wound from infection and mechanical damage;
- preventing moisture evaporation and loss of nutritional compounds through the open surface of the wound;
- more aesthetic appearance after healing of the wound surface.
The disadvantages of surgical intervention include:
- the possibility of rejection of the transplanted material (when transplanting your own skin, the risks are much lower);
- the likelihood of developing other complications;
- psychological discomfort of the patient (for example, if the donor material is cadaveric skin or tissue taken from an amputated limb).
Price
The cost of skin grafting is determined by various factors: the area of the damaged surface and the complexity of the specific operation, the type of materials and anesthesia used for transplantation, other pharmacological agents, the qualifications and professionalism of the surgeon, the location and reputation of the clinic. Depending on these factors, the price of the operation can range from 10,000 to 200,000 rubles.
Skin plastic surgery after a burn is not a complex or lengthy operation. But in some situations, graft engraftment after transplantation proceeds poorly, even if the donor material is one’s own skin. Detailed preparation for plastic surgery, correct calculation of the timing of its implementation, and appropriate care during the rehabilitation period are mandatory conditions for successful skin plastic surgery and help minimize the likelihood of developing postoperative complications.
Classification of grafts for transplantation
For skin transplantation, it is preferable to take material from the patient himself (autograft). If this is not possible, a living or deceased donor (allograft) is used. Sometimes doctors use the skin of animals, especially pigs. Developed clinics practice growing synthetic skin - an explant.
Depending on the depth of the lesion, the material for transplantation is divided into three types:
- thin - up to 3 mm. The biomaterial consists of the upper and additional layers of skin and has a small amount of elastic fibers;
- average - 3-7 mm. It consists of a mesh layer rich in elastic fibers;
- thickness - up to 1.1 cm. It covers all layers of the dermis.
The choice of material depends on the location of the burn, its size and the individual characteristics of the body.