Avdeyuk Elena Vladimirovna Dermatovenerologist, cosmetologist
Publication date: July 19, 2022
Review date: November 10, 2022
Indications and contraindications for use
Before undergoing an aesthetic correction procedure using the Sculptra™ implant, it is necessary to carefully collect an anamnesis and identify the presence of absolute and relative contraindications (such as acute or chronic skin diseases, signs of infection or inflammation in or near the injection site). Biostimulant Sculptra™ should not be used in patients with allergies to any of its components.
Patient profile for aesthetic facial correction using Sculptra™:
- Age over 30 years (in some cases, it is acceptable to use an implant from 18 years of age)
- Any aging morphotype
- Reduced tissue elasticity, sagging skin
- Thinning of the skin and subcutaneous tissues
- Loss of facial volume
- Lipoatrophy
- Tissue ptosis
- Facial asymmetry
- Atrophic scars after acne
- Significant weight loss or planned weight loss
Elimination, minimization of the effect
It will not be possible to eliminate or minimize the result if you are dissatisfied with the achievements. The filler is gradually removed from the tissues naturally. You can try to speed up the process with a series of physiotherapeutic procedures, increased physical activity, steaming the skin (actions that provoke the activation of internal processes). Usually this is not required.
The result of the filler, when done professionally by a cosmetologist, is soft. The doctor prescribes repeated interventions only after assessing current achievements.
Preparation and carrying out the procedure
According to the instructions for use of Sculptra™, the volume of sterile water for injection (SWI) for hydration of the lyophilisate is 5 ml. Currently, American and European expert consensus recommends using 7-8 ml of SVDI for Sculptra® restoration. And according to the Russian consensus of experts, there are advantages in reconstituting Sculptra™ lyophilisate in one 8 ml SVDI bottle. The lyophilisate must be reconstituted at room temperature at least 2 hours before the procedure (there are recommendations - 24–48 hours) to ensure the necessary hydration of the material. Do not shake the bottle during the hydration period. When the implant is ready for injection, shake it gently immediately before use until a homogeneous suspension is obtained. Before use, wipe the rubber stopper of the bottle with an antiseptic, then, using a sterile 18G needle, take the required amount of suspension into a disposable sterile syringe. Next, you can add 1-2 ml of a 2% lidocaine solution to the bottle, and gently mix the lidocaine solution with the suspension with rocking movements. Thus, you should get 9–10 ml of a homogeneous suspension, the liquid phase of which is represented by 8 ml of SVDI and 1–2 ml of a 2% lidocaine solution. It is not recommended to store the collected suspension in a syringe for a long time, as particles settle.
Another method for reconstituting Sculptra™ lyophilisate is also being discussed.
Baumann K. et al. in 2022
showed that the physicochemical characteristics of a PLLA suspension immediately after the addition of SVDI and vigorous shaking are comparable to those obtained by longer hydration of the product. The researchers reconstituted the Sculptra™ lyophilisate immediately before injection by sequentially adding SVDI (5 ml, then 3 ml) and mixing thoroughly. Clinical studies are currently being conducted to confirm laboratory test data. These studies are important for clinical practice, as there is often a need for a finished product immediately before a procedure.
Purchasing a product
Numerous online stores offering materials for aesthetic medicine sell Sculptra fillers. The price of a package of 2 bottles of lyophilisate is 12–15 thousand rubles. In terms of 1 ml of finished product, the price is considered as attractive as possible. We must not forget that the powder from the bottle is not used partially, and the time for using the finished substance is limited.
Important! Only a qualified physician should perform injections. Patients seeking to save money by purchasing the substance themselves are not advised to forget about this.
Application techniques in the facial area
Figure 2.
Facial areas recommended for use of the Sculptra™ implant
The Sculptra™ implant is injected subdermally, into the subcutaneous fatty tissue, since with superficial injections there is a risk of nodules at the injection site, which indicates an incorrect injection technique or an excessive amount of injected suspension. In some areas (temporal zone) it is recommended to administer the product under the SMAS, the superficial layer of the temporoparietal fascia, given the very thin layer of subcutaneous fat in this area (Fig. 2)
.
In some cases, the implant is inserted into the deep fat pads of the face (ROOF, SOOF, preperiosteal malar fat, Ristow's space, deep medial and lateral buccal fat) in the form of microboluses with a volume of no more than 0.2–0.3 ml, which, according to all experts , is a safe technique.
Massaging the insertion area to ensure uniform distribution of the implant is mandatory not only at the time of the procedure, but also in the next five days. This prevents the appearance of irregularities and the formation of nodules.
For injections, 26–27G needles with a length of 13–25 mm or 22–23G cannulas with a length of 38–70 mm are used.
For subdermal injections, a linear-retrograde needle technique is recommended. The required volume of suspension should be evenly distributed throughout the entire correction zone: on average, it is 0.1–0.2 ml/cm2. When using a cannula, the product is injected into the superficial fatty tissue (SAT) in a linear-retrograde fashion, in a fan pattern of 0.1–0.2 ml.
The Sculptra™ implant is not inserted into areas of high facial activity, in areas with thin skin and unexpressed subcutaneous fat, namely in:
- periorbital, including lacrimal and eyelid-buccal grooves;
- perioral, including the contour and red border of the lips;
- forehead and glabella area (eyebrow area);
- nose.
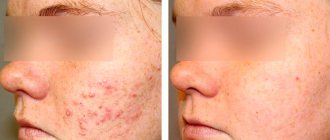
Photo 1.
A 65-year-old patient before and 6 months after the administration of 1 bottle of Sculptra™. Photo courtesy of D. Yu. Zakharov
Before
After
Patient reviews
The effect of Sculptra gel, according to patients, depends on the professionalism of the cosmetologist. In skillful hands, the product gives amazing results. The effect is often positive. There are no sharply negative opinions from patients.
The patient likes the results of Sculptra injections.
The patient highly appreciates the results of the drug Sculptra.
Adverse reactions
Adverse reactions from implantation, described in the instructions for use of Sculptra™, are non-specific and are associated with the injection procedure itself: short-term bleeding in the area of skin puncture, transient pain at the injection site, redness, ecchymosis, hemorrhage, mild swelling. These phenomena, as a rule (according to the clinical experience of Russian experts), resolve spontaneously within 2–7 days.
Other adverse reactions are mainly due to errors in the preparation and/or administration of the product. A characteristic element of incorrect insertion of the Sculptra™ implant is a nodule. The reason for the appearance of nodules at the injection site is most often a superficial injection, an excessive volume of the injected product or its incorrect restoration, an incorrectly selected area (that is, areas with weakly defined subcutaneous fatty tissue: lips, forehead, glabellar area, skin above the upper and lower lip) . It is also extremely important to instruct the patient to self-massage the injection areas for 5 days after the procedure.
Precautionary measures
Identification of contraindications that lead to refusal of the procedure helps to avoid complications. The number of strict restrictions is small:
- infectious, any acute disease;
- impaired blood coagulation;
- skin problems in the places where injections were planned;
- allergy to the components of the drug, other materials used in the work.
The presence of certain diseases in the anamnesis (various psychosomatic, endocrine, cardiovascular pathologies) leads to an individual decision about the possibility of intervention. The procedure is not performed during pregnancy, lactation, with the development of oncology, or with a predisposition to hypertrophic tissue healing. Some facts: previously performed surgeries, procedures (plasty, peeling, the presence of Botox, other fillers) may prompt a shift in the date of the procedure, a complete refusal of injections.
The manufacturer claims a low likelihood of complications after Sculptra filler injections. If the rules for working with the drug are violated, complications are possible for the patient:
- inflammation;
- necrosis;
- severe tissue discomfort;
- formation of “balls”, seals, seromas;
- gel contouring.
A doctor will help you find ways to solve problems. If symptoms are detected, contacting a cosmetologist is mandatory.
Combination with other aesthetic procedures
It is possible to combine Sculptra™ implant injections with hardware and other aesthetic correction methods in accordance with the protocols. If the zones and depth of injection do not coincide, then Sculptra™ can be used in one patient visit in combination with fillers, skinboosters, botulinum toxin A. If, after using the Sculptra™ implant, the patient is planning a laser therapy session, chemical peeling and other aesthetic procedures associated with active skin response, there is a possible risk of an inflammatory reaction at the implant site. Sculptra™ is administered after the skin has completely recovered from such procedures.
Features of the drug Sculptra (Sculptra)
The drug Sculptra (Sculptra) is an injectable implant based on polylactic acid. Polylactic acid is used in medicine for the production of absorbable suture material; its biocompatibility is beyond doubt. The lifting effect when using Sculptra increases gradually - after administration of the drug, there is a noticeable increase in tissue volume and a slight rejuvenating effect, due to the fact that the implant fills the voids of the intercellular space. The presence of the drug Sculptra in tissues activates the production of collagen and stimulates the processes of its own rejuvenation.
The effect of Sculptra is somewhat similar to the effect of fillers based on hyaluronic acid, but Sculptra is more effective, and this effect lasts longer.
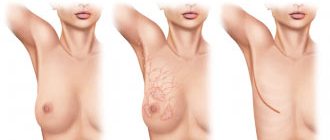
Using the Sculptra™ implant to correct extrafacial areas
According to international and Russian experts, the Sculptra® implant is indicated for the correction of sagging and atony of the body’s skin, changes in relief, tissue ptosis, local atrophy after liposuction, as well as stretch marks and cellulite.
Areas recommended for use of the implant: neck, décolleté, inner surface of the shoulders and thighs, axillary zone, supra-elbow and patella zone, abdomen, buttocks.
The use of Sculptra® for aesthetic correction of the breast area, dorsum of the hands, calf area and genital area is not recommended due to the lack of safety and effectiveness data and positive experience in these areas.
Before starting to use the Sculptra™ implant, it is important to consider the following aspects that may affect the result: the presence of significant ptosis and excess skin, lifestyle, excessive sun exposure, smoking and poor nutrition, menopause. All these factors can lead to a decrease in the body's individual response to the injection and require an increase in the number of sessions and/or the need to increase the total volume of the implant administered.
The use of the Sculptra™ implant for aesthetic correction of the body (the area below the edge of the lower jaw) always involves additional dilation, at least twice as large as the standard one. That is, after preliminary reconstitution of the Sculptra™ vial in 8 ml of sterile water for injection (WFI), immediately before injection, 2 ml of a 2% lidocaine solution and 6-10 ml of WFI are added to the suspension, so the final volume of the suspension is 16-20 ml. The concentration of PLLA with a total suspension volume of 20 ml is 0.75% (7.5 mg/ml). For mixing, it is convenient to use a 20 ml syringe to aspirate 8 ml of suspension from the bottle, 2 ml of a 2% lidocaine solution and 6-10 ml of SVDI. Using a sterile connector, transfer the contents into smaller syringes (1 or 3 ml) for injection. Typically, one bottle of Sculptra™ is used in one session per area equivalent to an A4 page. This recommendation applies to all areas of the body.
After the procedure, it is necessary to massage the injection area for several minutes. Patients are recommended to massage this area for 2 minutes 2 times a day for 5 days after the procedure. Another important post-procedure recommendation is to avoid physical activity and sun exposure for the first 24 hours.
Skin lifting typically requires 2-4 injections of the Sculptra™ implant at intervals of at least six weeks. In most cases, the first results appear within 2–3 months.
To maintain the effect, additional injection sessions are recommended every 12–18 months, depending on the degree of loss of skin elasticity.
Photo 2.
Improving the relief of the skin of the neck and décolleté, reducing the depth of wrinkles in a 45-year-old patient after the introduction of the Sculptra™ implant (2 sessions, 1 bottle each). Photo courtesy of I. P. Dmitrieva
Before
After 8 months
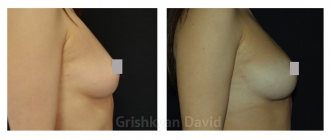
Photo 3.
Reduction of skin laxity, improvement of the relief of the inner surface of the shoulder in a 69-year-old patient after the introduction of the Sculptra™ implant (2 sessions, 2 bottles each). Photo courtesy of I. P. Dmitrieva
Before
In 6 months
Photo 4.
Treatment of cellulite and sagging skin of the buttocks. The result of inserting the Sculptra™ implant into a 45-year-old patient. 2 bottles were injected in 1 session. A subcision technique was used and simultaneous insertion of the Sculptra™ implant into the subcutaneous layer using a 22G cannula. Photo courtesy of I. P. Dmitrieva
Before
After 3 months
Healing period
After using the Sculptra filler, the following may appear on the face: redness, slight bruising, slight swelling of the tissues, puncture points often remain noticeable. The formation of moderate swelling and compaction under the skin is possible. Most side effects go away within 1–2 weeks.
No special skin care is required after Sculptra gel injections. After the needle prick wounds have healed, you can use your usual cosmetics. On the first day, it is advisable to refuse washing and other skin treatments. Cover hygiene is ensured by using an antiseptic.
To facilitate rehabilitation, minimize the possibility of complications, and obtain a predictable result, the doctor will warn against:
- applying makeup, visiting public places, swimming in public bodies of water until the punctures heal;
- tanning, steaming, physical activity from 1 week to a month;
- other procedures (Botox, intensive massage, injections of other substances, hardware effects).
If local compactions or “nodules” under the skin are detected, the cosmetologist will recommend carefully kneading the formations. For prevention, you can regularly (2-5 times a day) perform a light massage of the affected areas.
The result of using Sculptra filler is visible immediately after the procedure: the skin is smoothed, the tissues are filled. The mechanical action of the gel becomes noticeable. The delayed effect begins to be observed no earlier than after 1–2 months. The maximum positive effect develops after 6 months. The result is delayed for 2 years.
conclusions
Stimulation of neocollagenesis to restore the volume and biomechanical properties of soft tissues is a promising method for correcting age-related changes. Modern concepts and strategies of rejuvenation are not aimed at correcting a specific wrinkle, but at restoring the harmonious appearance of the face and body as a whole, improving the quality of the skin and returning it to a healthy, youthful appearance. Most patients are interested in techniques that provide long-lasting results. The use of the Sculptra™ implant both as “monotherapy” and in combination with other methods of aesthetic correction allows us to adequately respond to these challenges of the time.
Opinion of cosmetologists
Doctors often use Sculptra in their work. The substance has almost no direct competitors. The effect of the product is bright and long lasting. Considering negative reviews from colleagues and the possibility of working with more predictable products, cosmetologists do not always strive to use the substance.
The cosmetologist is sure that the effect of Sculptra gel is unpredictable.
The cosmetologist considers the drug Sculptra unsuccessful.
The cosmetologist is selective about prescribing Sculptra injections.
General characteristics
Sculptra is a drug with good bioavailability by the body. With its help, not only facial modeling occurs, but hips, breasts and buttocks are corrected. The gel also does an excellent job of eliminating age-related skin deformations.
The effectiveness of the product has been confirmed by considerable practice. In Britain alone there are about 17,000 successful procedures.
Composition of the drug
The filler is based on polylactic acid - it is produced in laboratory conditions through the synthesis of non-animal raw materials.
This method allows you to achieve maximum results in the biocompatibility of the filler with the human body. Previously, it was used in surgery as a self-absorbing suture material. When used in contouring, the drug has a different function - it promotes the production of natural collagen by the body itself.
Studies show that after just a couple of sessions, the formation of collagen fibers increases by 30%, and after a certain time, the dermal filler undergoes degradation and is completely removed.
The product is presented in dry form in the packaging. The ampoule contains:
- sodium carboxymethylcellulose (90 mg);
- non-pyrogenic mannitol (127.5 mg);
- polylactide (150 mg).
Manufacturer
Initially, the Sculptra filler had a different name “New Fill” and was developed to replenish lost volume in patients with AIDS. The production of the product was fully launched back in 1999.
The manufacturer of the drug is the French cosmeceutical company Sanofi Aventis. The product is also produced by an American concern in Sweden.