MAKING AN APPOINTMENT WITH A DOCTOR IN ST. PETERSBURG
+7 or +7
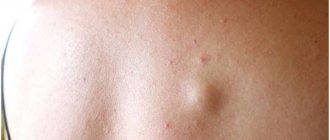
Basal cell carcinoma of the skin (BCC)
, or
basalioma
, is the most common type of skin tumor, occupying an intermediate position between benign and malignant neoplasms.
BCCs account for about 75-80% of all cases of so-called non-melanoma skin cancer, which is significantly less aggressive compared to melanoma
.
Basalioma rarely metastasizes even in comparison with other types of non-melanoma skin cancer (no more than 0.1% of cases) - its danger lies elsewhere. Balasioma can disfigure your appearance beyond recognition, causing local destruction of large areas of soft tissue, cartilage and bone.
Basal cell carcinoma accounts for approximately 32% of all cancers in the world, and epidemiological data indicate a steady increase in the incidence of basal cell carcinoma by 3–10% per year. In the United States alone, 4.3 million people are diagnosed with basal cell carcinoma every year, which is why it is so important to know everything about BCC.
Symptoms and signs of basal cell carcinoma
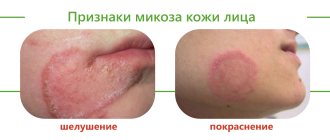
Basal cell skin cancer is usually located in prominent places - on the skin of the face, ears, neck, and less often - the scalp. Most often, basal cell carcinoma “chooses” the skin of the nose and eyelids. Sometimes he doesn’t disdain the body and limbs either, so be careful when examining yourself.
Clinical subtypes of basal cell carcinomas are classified by appearance. These may be formations similar to a papule with vascular veins, psoriasis, a mole or a scar. Over time, the tumor may grow and take on the appearance of an ulcer or non-healing wound, bleeding and peeling. BKK options:
| Type | Type description |
Solid (nodular) type of basalioma(50% to 80% of all basal cell carcinomas)Small, shiny, dense, almost transparent to pink nodules with telangiectasia, which are usually localized on the face. Nodular basaliomas may have crusts, abrasions, lichenoid or ulcerated elements. Over time, an untreated ulcer can penetrate into deep extradermal structures, down to the bone. | |
Superficial type of basalioma(15 to 30% of all basal cell carcinomas)In this type, the lesions are red or pink, with clear boundaries, thin papules and plaques that are difficult to distinguish from psoriasis or local dermatitis. Occurs in younger patients. Most often localized on the trunk and limbs (up to 60%). They can develop in combination with nodular basal cell carcinoma. | |
Pigmented type of basal cell carcinoma(from 6% to 50% superficial BCC depending on the patient’s phototype)Some superficial basal cell carcinomas may contain nests of melanin. In patients of European descent, only 6% of tumors contain an increased amount of melanin, but in dark-skinned people, about half of basal cell carcinomas are brown. Melanin is usually distributed unevenly. | |
Morpheoform type of basal cell carcinoma(5 to 10% of all basal cell carcinomas)Flat, scar-like, compacted plaques, flesh-colored or light red in color, with blurred edges and pronounced connective tissue stroma. In 95% of cases they are localized on the scalp and neck. They rarely bleed and practically do not ulcerate, but can develop in combination with nodular basal cell carcinoma. |
Fibrosing connective tissue diseases include a wide range of conditions characterized by limited areas of fibrosis involving varying levels of the dermis, subcutaneous tissue, and sometimes underlying soft tissue and bone. The most pronounced fibrosis of the skin is observed in scleroderma.
The term “scleroderma” can be literally translated as “thickening of the skin” and often serves as a collective term for a diverse group of diseases, the common feature of which is pathologically increased collagen formation. Scleroderma can be broadly divided into limited sclerosis and systemic sclerosis. Limited scleroderma is characterized by lesions of the dermis and subcutaneous tissue and the absence of clinical signs of a systematic process - Raynaud's syndrome, acrosclerosis and involvement of internal organs.
There are several classifications of this pathological process. According to the clinical classification of L. Peterson [1], the disease is usually divided into plaque, deep subcutaneous, bullous, linear forms and frontoparietal lesions of the “saber blow” type with/or without facial hemiatrophy.
D. Tuffanelli and R. Winkelmann [2] in turn identified three forms of limited scleroderma: plaque, characterized by limited sclerotic brownish plaques with a growth ring along the periphery of the element; linear, manifested as an elongated lesion with linear growth, and generalized - the most severe form of limited scleroderma, characterized by widespread skin lesions with multiple indurative plaques, hyperpigmentation and often muscle atrophy. Plaque and linear forms are the two most common types of pathological process, and only the linear form has been reliably demonstrated to be associated with damage to internal organs. Although conditions such as Pasini-Pierini atrophoderma, eosinophilic fasciitis, and lichen sclerosus are sometimes considered variants of focal scleroderma, such a distinction is rarely used [3].
The plaque form of scleroderma usually begins with the formation of an erythematous lesion or local edema, which over time becomes more indurated and acquires a whitish, yellow, or porcelain hue. During the active phase of the disease, the induced area may be surrounded by a lilac corolla of growth along the periphery. This is usually followed by a resolution phase, during which individual lesions become atrophic, hypo- or hyperpigmented, and there is no hair growth or sweating in the affected area [4]. Skin lesions are usually superficial with involvement of the dermis, however, with thicker plaques, adhesion to the underlying tissues is observed, which can lead to a decrease in sensitivity in the area. For lesions with minimal induration, the term “superficial scleroderma” has been proposed [5]. As morphological subtypes of plaque scleroderma, it is customary to distinguish guttate, bullous, keloid and deep forms, some of which are considered as separate variants of focal scleroderma within the Peterson classification.
The teardrop form is characterized by multiple small (less than 10 mm in diameter) and superficial lesions, predominantly involving the neck and upper half of the body, with less pronounced induration and a sharply limited margin. The rashes have a whitish tint and the clinical picture is similar to extragenital forms of lichen sclerosus, but with true guttate scleroderma, epidermal atrophy and follicular hyperkeratosis are weakly expressed. It is assumed that these diseases may have a similar pathogenesis and coexist [6, 7].
As already noted, in his classification L. Peterson [8] identifies bullous scleroderma as a separate morphological variant of the course of focal scleroderma, but the appearance of blisters has been described in all types of the disease. It is generally accepted that this phenomenon occurs due to the dilation of lymphatic vessels and the release of large basic protein by eosinophils. Some authors [9] suggest that the appearance of blisters may indicate the presence of concomitant lichen sclerosus.
The keloid (nodular) form is a rare variant of the course of plaque scleroderma, in which nodules resembling keloids form against the background of typical scleroderma lesions [10].
Linear scleroderma is characterized by strip-like foci of skin induration, often single and in 95% of cases located unilaterally, accompanied by pigmentary changes that can cross the lines of the joints and lead to contractures. This form of the disease most often develops in children and in most cases affects the limbs. The fibrotic process can spread to the subcutaneous fat, fascia, muscles and, in rare cases, even the meninges and brain. Damage to muscles and bones in 20% of cases leads to impaired growth and possible severe flexion deformities [11, 12]. The main features of the lesions are similar to those of the plaque form, however, the purple corolla is not noticeable or is noted along the growing edge. The linear form most often develops on the lower extremities, followed in frequency by the upper extremities, the frontal region of the head and the anterior surface of the torso. Many cases of linear scleroderma with lesions located along Blaschko's lines have been described, although in most cases the rash does not correspond to them [13, 14]. Very rarely, half of the body is involved in the pathological process - face, arm, torso, leg [15]. Most often, the lesions have a longitudinal location along the length of the limb or concentrically around the body, but sometimes the pathological process concentrically covers the limb or finger, in some cases leading to amputation, which is reminiscent of Angus disease [16].
In very rare cases, the disease affects the entire limb with the development of a pansclerotic process [17]. Disabling pansclerotic scleroderma of children is a rare, severe, mutilating form of the disease involving the dermis, subcutaneous fat, muscles and bones, most often developing before 14 years of age, although later cases have been described [18]. Superficial and deep sclerosis of the skin affects the trunk, limbs, scalp and face, while the tips of the fingers and toes remain intact. Claw-shaped deformation of the hands may be observed. Patients may walk on their toes due to Achilles tendon contracture. The onset of the disease is characterized by stiffness and arthralgia, which is subsequently followed by severe pain in the extremities due to damage to the cutaneous nerves. Raynaud's phenomenon is not typical for this process [19]. Pansclerotic scleroderma in children is associated with an increased risk of developing squamous cell skin cancer, especially in the presence of ulcers in the affected areas [20, 21].
Linear scleroderma developing on the face may appear as a red-brown streak or a single whitish atrophic lesion running vertically across the forehead, known as an “un coup de sabre.” The onset of the disease is characterized by tightening and thickening of the skin in the affected area. Subsequently, a lilac plaque develops, sometimes with pronounced telangiectasia on the surface, together with hyperpigmentation at the edge. As a result, a linear depressed groove is formed in the frontoparietal region, spreading to the skin of the scalp with the development of a focus of scarring alopecia, which may be preceded by graying of the hair. This process is more characterized by a paramedian location of lesions than a median location [22]. In severe cases, the lesion may extend lower to involve the cheeks, nose, lips and gums. Quite often, the progression of the process leads to atrophy of the corresponding half of the face with the development of its asymmetry. Rarely, fronto-parietal lesions can be located bilaterally and correspond to Blaschko's lines [23, 24]. When the orbital region is involved in the process, enophthalmos, eyelash loss, and myopathies of the external eye muscles can develop [25].
It is generally accepted that Parry-Romberg syndrome (progressive atrophy of the half of the face) is a severe segmental form of craniofacial linear scleroderma. In contrast to the “saber strike,” the primary lesion develops in the subcutaneous fatty tissue, muscles and bones without the development of skin sclerosis at any stage of the process [26]. Despite the fact that there is no induration or tightening of the skin, in some patients primary foci of skin sclerosis are formed, resembling a “saber blow” [22].
The deep subcutaneous form of scleroderma primarily involves the subcutaneous fatty tissue and underlying structures, such as the fascia. This term was first proposed in 1989 to describe single fibrotic plaques on the skin of the shoulders, back, neck with histological signs of fibrosis, hyalinization of collagen fibers and deep infiltrates of the dermis and subcutaneous tissue. The skin overlying the area may be hyper- or hypopigmented. Sclerotic plaques are ill-defined and have the appearance of a cobblestone pavement or pseudocellulite; later, as the disease progresses, a groove symptom may develop - a depression along the course of a vein or between muscle groups. However, it is believed that in most cases, deep scleroderma rarely progresses.
The generalized form of focal scleroderma is a condition in which idiopathic sclerosis of the skin is widespread. Diagnostic criteria for diagnosis are: four or more lesions with a diameter of more than 3 cm, regardless of whether they have a plaque or linear shape; involvement of two or more of seven body regions (head, trunk, right upper limb, left upper limb, anterior trunk, posterior trunk, right lower limb, and left lower limb) [27, 28]. Patients who do not meet these criteria should be diagnosed with plaque or linear form based on morphological features. The onset of the disease, as a rule, does not differ from the usual plaque form, and patients notice the appearance of similar lesions. In the early stages, a purple growth ring is noted around the whitish-purple lesions. The size of the lesions is much larger than in the plaque form (several centimeters in diameter). Typically, lesions appear on the trunk and gradually enlarge with the formation of new plaques during the first 2 years of the disease. Most often the upper half of the torso, mammary glands, abdomen and upper thighs are involved in the process. A rare variant of the generalized form is almost universal scleroderma involving the entire body from head to feet, but unlike systemic sclerosis, patients do not have sclerodactyly, Raynaud's phenomenon, changes in the capillary bed of the nail bed and damage to internal organs [29].
Systemic sclerosis is a multisystem disease characterized by vascular abnormalities, connective tissue sclerosis, atrophy, and autoantibodies. The diagnostic criteria were developed by the Scleroderma Subcommittee of the American Rheumatism Association and have been widely accepted throughout the world. Patients must have one major criterion (thickening, tightening and induration of the skin of the fingers and areas above the metacarpophalangeal and metatarsophalangeal joints, involving the limbs, neck, face and trunk) or at least two minor criteria, which include sclerodactyly, depressed scars on finger pads and bilateral pneumosclerosis of the basal parts of the lungs [30]. These criteria have a sensitivity of 97% and a specificity of 98%, however, difficulties may arise in patients with scleroderma-like conditions due to occupational hazards, in particular with exposure to silicon and vinyl chloride, who may often have two minor criteria for systemic sclerosis and other similar symptoms [31] .
Systemic sclerosis is usually classified into three forms: limited cutaneous systemic sclerosis, diffuse cutaneous systemic sclerosis, and crossover syndromes that combine manifestations of scleroderma and other rheumatic diseases [32]. Although there are clear similarities between focal scleroderma and systemic sclerosis, most notably cutaneous fibrosis, marked differences remain between the cutaneous and systemic manifestations of the two disease groups. First of all, with focal scleroderma, with the exception of the linear variant, systemic lesions are extremely rare. Skin manifestations also differ between the two groups. Plaques in focal scleroderma have clearer outlines and are more heterogeneous than in systemic sclerosis. Heterogeneity is observed both within the plaque itself (sclerosed center and more inflammatory outer edges) and between different plaques in the same patient. Anatomically, focal scleroderma can occur on any part of the body, while the favorite areas of localization for systemic sclerosis before the appearance of more widespread rashes are the perioral zone and hands. The limited form of systemic sclerosis is characterized by symmetrical, progressive fibrosis of the skin of the distal parts of the extremities, sometimes limited to only the fingers. Compared with plaques in focal scleroderma, fibrotic phenomena in systemic sclerosis are less pronounced, and developed telangiectasia is noted. The transition from the edema stage to the sclerotic stage can take 5-15 years, and in some patients acrosclerosis may not develop [33, 34].
The diffuse form of systemic sclerosis is characterized by the formation of widespread symmetrical fibrosis involving the upper and lower extremities, trunk and face. The time interval between the edematous and sclerotic phases is shorter than with the limited form. Very often, ulcerative defects form on the fingertips and bone protrusions, and often such patients experience calcification, which can serve as a source of ulcerative defects and secondary infection.
If we assume a connection between focal scleroderma and systemic sclerosis (considering these two diseases as two final stages of a single pathological process), then there should be a certain proportion of patients in whom the focal form [35] develops into systemic sclerosis. However, isolated cases of transition from focal scleroderma to systemic sclerosis or the coexistence of the two processes have been reported [36].
Lichen sclerosus is a chronic dermatosis characterized by small whitish atrophic foci that appear on any area of the skin. Lichen has both genital and extragenital forms, which can occur separately or simultaneously. It is generally accepted that damage to the vulva can lead to the development of squamous cell carcinoma, but the involvement of additional factors in this process has not been fully studied. Skin rashes are usually asymptomatic and develop on the trunk, especially in the upper parts, around the navel, neck, axillary areas and on the flexor surfaces of the wrists [37]. The elements of the rash are small, ivory or porcelain-colored, shiny, sometimes translucent, reminiscent of mother-of-pearl. The rash may be widespread, involving almost the entire torso, and may follow Blaschko's lines [38]. On the surface of the papules, dilated ducts of the sweat and sebaceous glands are noted, which often contain horny gum-like plugs of a yellowish color, clearly visible during dermatoscopy. Sometimes the process is accompanied by the appearance of blisters, telangiectasia and purpura [39]. In later stages, as atrophy progresses, the surface of the rash becomes wrinkled and may sink. Papules can merge into plaques resembling scleroderma, but individual elements of lichen sclerosus are almost always present.
Genital rashes in women may be limited to the labia majora, but usually involve the labia minora and may eventually lead to obliteration of the vaginal opening. Involvement of the perianal and perivaginal areas often results in hourglass or figure eight shaped lesions with minimal involvement of the perineum. Against the background of friction and weeping, the rashes often macerate and outwardly resemble abrasion. Sometimes the process is accompanied by the appearance of vesicles and blisters with hemorrhagic contents [40]. Patients with lichen sclerosus may develop premalignant and malignant changes, with the incidence of squamous cell carcinoma being 5% [41]. Typically, carcinoma develops in the area of sclerosis, on the anterior surface of the vulva. The role of human papillomavirus in malignancy cannot be ruled out. Strains of human papillomavirus associated with carcinogenesis were found in women with lichen sclerosus [42].
Genital involvement in men is usually limited to the glans penis and preputial sac. Involvement of the trunk is much less common, and the scrotum is extremely rare. The initial manifestation of the disease may be a sclerotic ring along the edge of the preputial sac. Symptoms of lichen may include acquired phimosis or recurrent balanitis, as well as itching, burning and pain during erection [43]. The foreskin becomes sclerotic and inactive, the glans penis and the inner layer of the foreskin become shiny, bluish-white, sometimes with a significant amount of telangiectasias. Due to displacement of the urethral orifice, compression can develop, and lichen sclerosus is observed in 14% of patients with urethral stenosis [44].
Considering the high incidence of lesions of the mucous membranes of the genitals, the relatively rare lesions of the oral mucosa remain surprising. Rare cases have been observed in patients with a generalized form of lichen.
Idiopathic Pasini-Pierini atrophoderma is a form of dermal atrophy of the skin, manifested by one or more well-defined hyperpigmented, slightly depressed plaques, usually arising on the back. Whether atrophoderma is a non-sclerotic, primarily atrophic variant of focal scleroderma remains a matter of debate [45]. The connection between the two diseases is supported by the pronounced similarity in the clinical and histological patterns of atrophy observed in regressive plaques in scleroderma. The presence of antibodies to Borrelia burgdorferi has been reported in patients with atrophoderma [46]. A number of researchers have observed the simultaneous appearance of foci of plaque scleroderma, lichen sclerosus and atrophoderma in one patient, but appearing on different parts of the body. In studies by Y. Yokoyama et al. [47] demonstrated that glycosaminoglycans isolated from lesions of Pasini-Pierini atrophoderma differ from those in typical plaques of focal scleroderma. Also, the difference in the course and outcome of the process suggests a different genesis of the two processes. The disease usually begins in adolescence as a slightly swollen erythematous lesion, most often appearing on the body in the lumbosacral region. The lesions are represented by multiple symmetrical plaques of ovoid or irregular shape, ranging in size from a few centimeters to large areas. The skin surface is visually normal, without induration or sclerosis. The edges of these areas are clearly defined, often described as abruptly ending, resembling a "cliff", occupying 1-8 mm in depth, giving the lesion the appearance of an inverted plateau. When there are several lesions, they resemble Swiss cheese in appearance. The skin surrounding the areas is not changed, the growth corolla characteristic of scleroderma is absent [48].
Sclerotic skin changes can occur in a number of conditions other than scleroderma or systemic sclerosis.
Eosinophilic fasciitis (Shulman syndrome) is a rare focal fibrosing disease of the fascia, a scleroderma-like syndrome, and appears to be a distinct entity, although it is sometimes considered to be an early onset of systemic sclerosis, linear scleroderma, or in combination with other connective tissue diseases. . The main signs of this pathology are thickening of the fascia in combination with eosinophilia, increased erythrocyte sedimentation rate and hyperglobulinemia. Clinical signs are acute pain, swelling, and tenderness of the distal extremities, which become induced. There is limited mobility of the hands and feet. Eosinophilia is observed in 70% of cases and is usually associated with an increased erythrocyte sedimentation rate and hyperglobulinemia [49, 50]. In eosinophilic fasciitis, Raynaud's phenomenon and visceral involvement are not usually observed. In the acute inflammatory stage, skin manifestations of the disease include erythematous swelling that does not subside with pressure. Subsequently, these manifestations are replaced by induration and fibrosis. The skin over the affected area is dense, fused to the underlying tissues. Sometimes “orange peel” type changes or a philtrum symptom are formed in the lesion due to the retraction of the veins. Skin manifestations are usually symmetrical. The areas most commonly involved are areas of the upper limb above and below the elbow joint and areas of the lower limb above and below the knee joint. 50–75% of patients develop contractures involving the elbows, wrists, ankles, shoulders and knees [51].
Nephrogenic systemic fibrosis is a scleroderma-like fibrosing disease [52]. All patients had renal failure. The precipitating factor for this disease is the use of gadolinium-based contrast agents for imaging, metal replacement in gadolinium chelates, which apparently leads to the deposition of free gadolinium in the dermis and other organs [53]. Rashes with nephrogenic systemic fibrosis have the appearance of erythematous papules merging into brownish plaques, which creates an “orange peel” appearance. The skin involved in the pathological process becomes dense and woody. The clinical picture resembles eosinophilic fasciitis. The rashes are symmetrical and usually form on the limbs and torso. Such patients can very quickly develop contractures, which leads to disability [54].
Pseudosclerotic changes can be seen in rheumatoid arthritis and acrodermatitis atrophicus, a disease common in Central and Eastern Europe that resembles scleroderma in appearance and is a late manifestation of Lyme disease. The process is characterized by the appearance of painless dark red nodules and plaques on the skin of the extremities, developing most often on the feet and legs, less often on the hands and forearms, slowly (over several months and years) spreading centrifugally, leaving central areas of atrophy. These same areas may have had chronic migratory erythema several years earlier [55]. Sometimes there is a spread of the lesion involving large areas of the body and face. Marginal growth may continue even when the central areas have entered an atrophic phase, in which the skin becomes flabby, hairless, tissue paper-like, pigmented, sometimes with symptoms of poikiloderma [56].
Scleroderma-like changes can be observed in a number of metabolic disorders, in particular in porphyria cutanea tarda and phenylketonuria, in which these symptoms develop in the first year of life [57, 58]. There are also reports that skin fibrosis may be associated with exposure to a number of chemicals.
1-6% of workers who come into contact with polyvinyl chloride, especially reactor cleaners, develop vinyl chloride disease, in which, in addition to symptoms of numbness, soreness, burning, pigmentation disorders of the skin of the fingers and hands, and to a lesser extent the feet, as well as general symptoms, skin thickening may be noted limbs, torso and face. The terminal phalanges are shortened with deformation of the nails. Telangiectasias appear on the face, reminiscent of those in systemic sclerosis. There may also be difficulty opening the mouth, although the “pouch-purse” symptom is not typical for this disease. It is usually extremely difficult to distinguish systemic sclerosis and vinyl chloride disease clinically [59]. Similar impairments have been reported following exposure to perchlorethylene, a solvent used for dry cleaning of clothing [60]. Exposure to trichlorethylene may be associated with scleroderma even after a single but long-term exposure [61]. The results of a study conducted in Eastern Europe showed that 28% of patients with systemic sclerosis had previous exposure to organic solvents.
Thus, fibrosing skin diseases are represented by a wide range of dermatoses, accompanied by sclerotic changes in the skin, developing both idiopathically and under the influence of external factors, sometimes leading to severe changes in other organ systems. Often the diagnosis is based on clinical symptoms. Knowledge of the main signs, including the localization of lesions, allows the clinician with a high degree of probability to make the correct diagnosis and determine further tactics for managing the patient.
Causes of degeneration of basal keranocytes
The tumor received the name “basal cell carcinoma” because of the similarity of its cells to the cells of the basal layer of the skin - the lowest layer of the epidermis. It is not surprising, since basalioma develops from keratinocytes just near the basal layer.
Epidermal stem cells, which exist in the body throughout its life, contribute to the constant renewal of the skin. A genetic malfunction in the cell division mechanism leads to the appearance of neoplasms. Damage to a cell's DNA can be caused by a number of reasons.
The main and most common cause of basal cell carcinoma is exposure of exposed skin to direct sunlight or UV radiation in a solarium. It is no coincidence that the favorite place for basal cell carcinoma is the human face, which is exposed to UV radiation more often and longer.
Peculiarities of skin structure and acne development
Human skin is a heterogeneous tissue. It has 3 layers:
- The epidermis is the top layer of skin in contact with the environment. It, in turn, consists of several more layers, the top of which is called the horny or outer layer. It is formed by cells that have lost their nucleus and other organelles and have a strong cell membrane called corneocytes, which are dead keratinocytes.
- The dermis is the middle and most important layer of the skin, which is responsible for all its functioning. After all, it is in it that blood vessels and numerous nerve endings are concentrated. Moreover, it is in the dermis that fibroblast cells are responsible for the tone and youth of the skin, synthesizing collagen and elastin fibers. It also contains sebaceous, sweat glands and hair follicles.
- Hypodermis or subcutaneous fat is the lowest layer of skin, represented by loose connective tissue and fat cells called adipocytes. Its thickness directly depends on the area of the body, gender and nature of nutrition. Therefore, on the face, subcutaneous fat is poorly expressed, but on the hips, especially in women, it is well expressed.
From the point of view of considering the issue of the development of acne, dermatologists and dermatocosmetologists are most interested in the dermis, or rather the activity of the sebaceous glands and other appendages of the skin, i.e., sweat glands and hair. The sebaceous glands are responsible for the synthesis of sebum, also called sebum. It is necessary to retain moisture in the skin, protect it from ultraviolet rays and the penetration of pathogenic microorganisms, and also to bring antioxidants to the surface of the skin and prevent oxidative damage. The functioning of the sebaceous glands is regulated by hormones of the hypothalamus, pituitary gland and adrenal cortex, the work of which is in direct relationship with the activity of other endocrine glands. Therefore, any changes in hormonal levels lead to the functioning of the sebaceous glands being disrupted in one direction or another.
It is to the sebaceous glands that we owe such an unwanted shine to the skin of the face in the so-called T-zone: on the forehead, nose and chin.
Often, hormonal fluctuations provoke more active production of sebum, which is called seborrhea. It is classified as a separate disease, the presence of which can be indicated by:
- enlarged skin pores, including the formation of comedones (popularly called blackheads);
- oily or dry flaking skin;
- rapid contamination of hair, which leads to the need to wash your hair much more often, including daily or even more often.
Hormonal disorders that provoke the development of seborrhea also lead to changes in the composition of sebum. As a result, the content of androgens (male sex hormones) increases and the amount of estrogens (female sex hormones), as well as linolenic acid, decreases. Sebaceous gland receptors are sensitive to androgens. Therefore, an increase in their number provokes more active production of sebum or sebum.
Both men and women produce both androgens and estrogens in their bodies, but in different quantities. Moreover, androgens are precursors of estrogens, i.e., the starting compounds for the synthesis of female sex hormones.
Linolenic acid is responsible for maintaining a slightly acidic environment on the skin, which is an effective prevention of the penetration of pathogenic microorganisms into it. Therefore, when the content of this unsaturated organic acid in sebum decreases, it becomes alkalized. This leads to a decrease in the barrier properties of the skin and the creation of favorable conditions for the development and reproduction of various microorganisms on its surface and penetration from the inside of the skin.
Causes of acne development
Activation of sebum synthesis, changes in its composition, in particular a decrease in the concentration of linoleic acid, is the main reason for the development of inflammatory processes in the skin, i.e., the occurrence of acne.
In the course of research, it was found that acne is most often provoked by:
- anaerobic lipophilic corynebacteria, in particular Propionibacterium acnes;
- aerobic bacteria, including Staphylococcum epidermidis;
- lipophilic fungi Pityrosporum ovale et orbiculare.
The most dangerous from the point of view of acne development are Propionibacterium acnes. These microorganisms are capable of synthesizing a variety of enzymes, including lipase, which destroys the walls of hair follicles.
The main problem is that all these microorganisms are a component of the normal microflora of the skin of the face, i.e. they are constantly present on it and in the normal state of immunity, the activity of the endocrine glands, and metabolism, their number is strictly regulated and cannot cause harm or provoke the development of diseases. Therefore, acne can be a consequence of various disorders in the body, i.e., the influence of endogenous causes, including decreased immunity and hyperandrogenism, i.e., increased synthesis of androgens (testosterone and dihydrotestosterone). Therefore, the disease often occurs when:
- puberty;
- endocrine diseases, in particular diseases of the ovaries and testicles, adrenal glands, pituitary gland and hypothalamus;
- obesity;
- premenopause and menopause;
- hyperkeratosis;
- gastrointestinal diseases;
- suffering from severe infectious diseases;
- long-term stress, chronic fatigue syndrome.
Often the tendency to develop acne is inherited.
But the reasons for the development of acne can also lie in the action of external causes, which include the negative influence of sunlight. This is due to the fact that with active exposure to ultraviolet radiation, the skin's immunity sharply decreases, i.e. its ability to suppress the growth and reproduction of microorganisms living on it. As a result, the bacteria and fungi listed above are able to multiply uncontrollably and form colonies around the sebaceous glands, as they feed on the secretion they produce - sebum.
Acne also often occurs in bodybuilders of both sexes. This is due to the fact that in order to achieve their goals, they are forced to eat high-calorie foods and often take anabolic steroids. Such drugs contain substances that are derivatives of testosterone. Therefore, their use for a long period of time leads to changes in hormonal levels and the development of seborrhea, and then acne. Such cases have even been identified as a separate form of acne - bodybuilding acne.
If women take anabolic steroids, in addition to acne, they may experience a decrease in voice tone, male-pattern hair growth, menstrual irregularities, even amenorrhea. But in both cases, bodybuilders risk decreased fertility.
Pathogenesis
In its development, the disease sequentially goes through a number of stages:
- excessive secretion of sebum;
- the occurrence of follicular hyperkeratosis, which consists of an increase in the thickness of the stratum corneum of the epithelium in the area of the mouths of the hair follicles, which leads to the formation of comedones;
- proliferation of microorganisms in clogged hair follicles, as they feed on the sebum produced;
- an inflammatory process that becomes the body’s reaction to the accumulation of metabolic products of microorganisms (can be observed on the surface of the skin or in its deep layers) and manifests itself in the formation of red papules, pustules, and nodules.
Often, as the duration of the disease increases, the number of inflammatory elements increases. In this, a direct role is played not only by blockage and damage by microorganisms to other sebaceous glands, but also by the rupture of their walls as a result of the accumulation of sebum and its distribution in the thickness of the skin.
Risk factors
The main non-modifiable risk factor for developing basal cell carcinoma is age.
.
Approximately 80% of patients are actually over 60 years of age
, and rarely - under 20 years of age. Men are more susceptible to BCC than women and are 30% more likely to experience a recurrence, especially within six months of the first lesion being identified and/or if they are over 65 years of age.
Other factors that increase the risk of developing basal cell carcinoma:
- Etiological
:- prolonged exposure to direct sunlight, in the mountains or southern regions;
- frequent exposure to artificial UV rays in a solarium.
- Exogenous
:- X-ray and radioactive radiation;
- exposure to carcinogens on the body (skin contact with arsenic, harmful working conditions);
- reduced immunity due to organ transplantation or HIV infection;
- the presence of severe sunburn in childhood, wounds, scars.
- Genetic
:- Phototypes I and II according to the Fitzpatrick scale
; - nevus of the sebaceous glands of Jadassohn;
- basal cell nevus syndrome, or Gorlin syndrome (leads to multiple BCCs);
- xeroderma pigmentosum;
- Bazex syndrome (can lead to early development of multiple BCCs);
- generalized basaloid follicular hamartoma syndrome;
- Rombo syndrome;
- Happle-Tinschert syndrome.
Depending on the clinical picture, they are usually divided into 3 types:
Benign
(atheroma, hemangioma, lymphangioma, lipoma, papilloma, mole, nevus, fibroma, neurofibroma)
They do not pose a threat to human life, but if poorly placed or large in size, they can cause disturbances in the functioning of other systems and/or organs of our body. Under external influences they can sometimes transform into malignant neoplasms.
Malignant
(basal cell carcinoma, melanoma, sarcoma, liposarcoma)
They grow quickly and aggressively, penetrating into surrounding tissues and organs, often with the formation of metastases. The prognosis of such diseases is often unfavorable, given the difficulty of curing them and the tendency for frequent relapses, and in some cases, active metastasis leads to death if vital organs are irreversibly damaged.
Borderline or precancerous skin conditions
(senile keratoma, xeroderma pigmentosum, cutaneous horn, Bowen's dermatosis)
Formations, the tissues of which, under the influence of hereditary or current causes, have changed, having the potential to degenerate into malignant tumors.
Diagnosis of basal cell carcinoma
The diagnosis is made based on histological examination
. Differential diagnosis of basalioma is carried out with:
- molluscum contagiosum,
- hyperplasia of the sebaceous glands,
- amelanoma
, _ - intradermal melanocytic nevus,
- Merkel cell carcinoma,
- trichoepithelioma,
- squamous cell carcinoma
- keratoacanthoma,
- inflammatory dermatoses (psoriasis and eczema),
- actinic keratosis,
- Bowen's disease
- morphea (focal scleroderma),
- dermatofibrosarcoma,
- scar.
Treatment of basal cell carcinoma
Like any other treatment, it is carried out with the goal of completely removing the tumor, as well as preserving the functions and appearance of the diseased organ. Because basal cell carcinoma usually occurs on exposed skin, primarily the face, cosmetic surgery may be required.
Removal of basalioma, depending on the characteristics of the tumor, can be carried out using different methods:
- curettage and electrocoagulation
; - surgical removal of the tumor with a margin of 1 to 4 mm;
- excision with complete control of peripheral and deep boundaries;
- Mohs micrographic surgery with sections up to five microns (if the tumor is localized on the face, in case of relapse and its high risk);
- cryodestruction;
- laser therapy;
- chemotherapy using chemotherapy drugs for external use (ointments with 5-fluorouracil (5-FU), imiquimod);
- intralesional injections of anti-inflammatory drugs;
- radiation therapy (if surgical treatment is not possible or as adjuvant therapy);
- radiotherapy for patients with contraindications to surgical treatment, topical and photodynamic therapy.
For patients with metastases, EGFR inhibitors (cetuximab and panitumumab) can be used.
Prognosis for basal cell carcinoma
Most basal cell carcinomas are quickly identified and completely cured. Cases where patients died due to tumor growth and damage to vital structures are rare, so a formal staging system is not used in most cases of BCC.
Clinical risk factors for BCC are:
- anatomical location and size of the tumor;
- clarity of the boundaries of basal cell carcinoma;
- type of tumor lesion (primary or relapse).
According to statistics, relapse of BCC disease is observed in 25% of patients: almost a quarter of those who have had a history of basal cell carcinoma develop a new basal cell carcinoma within 5 years after the appearance of the first tumor. Therefore, patients with a history of basal cell carcinoma should undergo an annual examination of all skin areas.
Scientists have noticed that most often the relapse of BCC occurs on the skin of the face, rather than carcinomas with primary localization on the trunk and extremities. Obviously, this is due to the fact that it is the face that is most unprotected from the effects of external factors harmful to the skin. In 1983, the concept of a “face mask” was proposed to designate high-risk skin areas.
Here are some more characteristics that help distinguish high-risk basal cell carcinomas:
- subtypes described by the term “aggressive histological pattern”;
- tumor size is more than 1 cm in diameter on the head and more than 2 cm in diameter on the body;
- tumor invasion into nervous and vascular tissue;
- lymph node involvement or metastases.
Clinical picture
Visually: small reddish rashes, “goose bumps”, dense nodules are localized at the very base of the hair follicle.
to the touch .
Localization : most often on the shoulders, hips, face and buttocks.
In the generalized form of follicular hyperkeratosis, extensive damage to the trunk and extensor surfaces of the extremities is observed.
Diagnostics:
The diagnosis is made by a dermatologist based on a visual examination; specialized techniques are not required.
It is necessary to distinguish follicular hyperkeratosis from acne. In the case of hyperkeratosis, the rashes on the face are dry, rough to the touch, small and uniform in size.