Allergan Natrelle has long been one of the most popular implants in Russia: many women underwent breast augmentation with them.
How come market leader Allergan suddenly decided to recall one of its best products, Natrelle? It is known that these endoprostheses have undergone repeated testing and have long since gained “authority.”
It turned out that new information came from the US Food and Drug Administration. There have been documented cases of cancer development after installation of Natrelle implants.
Should Allergan Natrelle implants be removed even if there are no complaints? This question has become very relevant for many patients today.
A few facts about Allergan Natrelle implants
- The basis of the composition is a cohesive gel.
- Macro-textured shell with a rough surface.
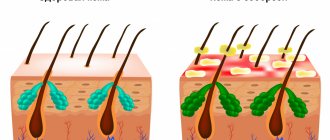
- The peculiarity of Natrelle implants was that after their installation, after some time, the patient’s own tissues grow into their shell. This ensures a secure hold and a lifetime of service.
According to research results, it is this feature of the implant shell that leads to the development of a dangerous complication.
Allergan. Brand history
19/09/2014
The innovative spirit that permeates Allergan today was sparked by the company's pharmacist founder, Gavin S. Herbert. In 1948, Mr. Herbert was already a successful owner of a chain of drugstores in Los Angeles, but his entrepreneurial talent was awakened in him by his close friend, chemist Stanley Bly, who turned to to him with the idea of creating antiallergic nasal drops containing the antihistamine neoantergan. They set up a small laboratory on the balcony of Mr. Herbert's pharmacy to create a solution they called Allergan Nasal Drops. In response to a request from an ophthalmologist friend, Mr. Herbert and Mr. Bligh created a different formulation of eye drops to treat allergic conjunctivitis (inflammation of the eyes). The result was the first antihistamine eye drops in the United States. Over the years, Allergan has achieved success by meeting the needs of its consumers. Allergan eye drops launched Mr. Herbert's successful career in ophthalmology and the founding of Allergan Pharmaceuticals, Inc. in 1950 to continue its efforts in the research and development of new therapeutic agents. Mr. Herbert continued to direct the company's efforts to develop and create new drugs for specialty areas, working closely with physicians and the scientific community to make drugs more effective. By 1953, Allergan had developed a number of new drugs, such as the first cortisone eye drops to treat allergic inflammation and the first steroid decongestant. In 1957, Mr. Herbert turned the company over to his son, Gavin Herbert Jr. Like his father, he continued to listen to the needs of doctors and patients and consult with specialists and researchers, guiding the development of innovative drugs in areas where there were unmet needs.
In terms of changes over the years, Allergan became a publicly traded company in 1970, merged with SmithKline Beckman in 1980, and was reinstated as an independent company in 1989. In July 2002, Allergan's ophthalmic surgical and contact lens care businesses were spun off to form a new company, Advanced Medical Optics, in 2005. In March 2006, Allergan acquired Inamed Corporation, which strengthened Allergan's position in high-growth markets as a pioneering biomedical company and created a global leader in the aesthetics business. All of this has contributed to Allergan, Inc., as we see it today under the leadership of David Pyott, who continues to inspire the company by adhering to the original principles laid down by the founders to quickly and sensitively respond to customer needs, which is the essence of the company's customer-centric business.
Today Allergan, Inc. Headquartered in Irvine, California, is a global diversified company with leading product portfolios in the areas of ophthalmology, neurology, medical dermatology, aesthetic medicine, breast augmentation, obesity surgery and urology.
Selling its products in more than 100 countries, Allergan meets the needs of its customers in various areas of medicine, improving the quality of life of patients.
Allergan worldwide and generated 2010 sales of more than $4.8 billion.
Do Allergan Natrelle implants need to be removed?
Some patients have already begun to sign up for surgeries to get rid of dangerous endoprostheses in order to avoid possible risks to health and life. However, experts report that in Russia there have only been two cases recorded in which lymphoma began to develop after breast augmentation with these implants.
What is the risk?
Plastic surgeons remind us that the risk is not that high. The probability of developing cancer is only 1:25,000.
Statistics are reassuring
In recent years, several dozen plastic surgeries for breast augmentation using Natrelle implants have been performed in our country. Most patients do not observe suspicious symptoms of the disease and feel well even several years after mammoplasty.
Quality checking
Implants used in our country always undergo strict safety testing. Allergan Natrelle breast implants were FDA certified (USA). In addition, after a series of tests, they were accepted in European countries and in Russia.
The implants have been recalled, but...
Allergan is a reputable manufacturer of implants on the global market. It's surprising that Natrelle had to be completely withdrawn from sales. The company decided to recall these products and confirmed that there is a possible health risk.
However, surgeons and many other experts urge their patients not to panic and not to rush to remove endoprostheses without a doctor's testimony.
An updated training center Allergan Medical Institute opened in Moscow
Secret of the mission
Everyone around the world has their own vision of development.
The company is one of the few that relies on training programs for cosmetologists and considers this an important part of its mission. And this is understandable, because Allergan, being one of the leaders in the field of aesthetic medicine throughout the world, has a portfolio of innovative drugs with one of the most highly studied quality and effectiveness profiles. According to the company, the foundation of the industry is not only original drugs, but also the specialist doctors who work with them - those whom thousands of people trust every day with their individuality, beauty and health. Allergan launched training programs for Russian cosmetologists back in 2012. During this time, the company supported more than 240 independent conferences and congresses on aesthetic medicine, and conducted more than 3 thousand master classes for cosmetologists from all regions of Russia. Every year, more than 2,500 physicians improve their professional level through training at the Allergan Medical Institute®.
Nadezhda Vischipanova , head of the Frau Klinik cosmetology department, noted , doctors are ready to learn new things and are ready to introduce innovations into their standard procedures. But the main thing that professionals value is working with proven, high-quality techniques, which allow them to compare favorably with the masters of the cheap and unsafe for patients “gray” segment.
And this is just the beginning. After several months of renovation, the company not only opened an updated training center equipped with the most modern equipment, but is also ready to offer several areas of educational programs designed for cosmetologists with different levels of training.
“Today we are pleased to open the doors of our updated Allergan Medical Institute® training center to once again share our expertise and knowledge, helping cosmetologists actively develop their professional skills and competencies,” said Mark Wilson, CEO in Russia, at the grand opening of the training center .
Let us remind you that it is globally represented in more than 100 countries, where it is actively developing its competencies in seven therapeutic areas. At the same time, the company invests about 10% of its revenue in scientific developments: for example, in 2022, the total investment volume exceeded $1.5 billion. The new product launch strategy involves annual drug launches in order to expand access to global innovations.
“We have a strict quality control system in place at all of our production sites. No matter where you are in the world, you can be confident in the quality of Allergan products,” added Mark Wilson .
Lighting up the stars
Nina Nasledukhova, director of the Aesthetic Medicine business unit of Allergan in Russia .
The company also has its own approach to training specialists in the aesthetic industry. The entire concept of professional training is based on active interaction with doctors. In this model, the doctor acts not only as a student, but rather as a partner, whose opinion is heard and taken into account in the further development of new training programs and master classes.
“Professional training of cosmetologists is one of the key indicators of the company’s professional development. And in this direction we intend to remain leaders. Our task is to ensure that everything we do in the field of vocational training is head and shoulders above what others do,” said Nina Nasledukhova .
Every year, Allergan makes significant investments in training and educational programs for doctors. In 2022, the company has already supported more than 50 congresses and conferences at both international and national levels. By the end of this year, it is planned to conduct more than 450 master classes, while increasing the number of doctors trained at the Allergan Medical Institute® to 3 thousand per year.
“We are using the capabilities of our training center so that Russian cosmetologists can gain wide access to the best world practices and trends in the field of cosmetology,” concluded Nina Nasledukhova .
In the near future, Allergan has plans to launch a large-scale online project: the digital platform Allergan Medical Institute® - AMI Digital, which will allow both beginners and experienced cosmetologists to constantly improve their professional level, wherever they are.
Moreover, when implementing this project, the company could not resist: “The system is built in such a way that for each user, depending on his current knowledge and competencies, his own personalized training plan will be developed for the most effective development in the field of aesthetic medicine. AMI Digital will allow you to gain access to many new training modules, webinars with the participation of international speakers, ask questions to Allergan experts and much more,” said Ivan Melnik .
Focus on freshness
At the gala event dedicated to the opening of the new educational center, many partners and friends of the company gathered, who have been fruitfully cooperating with Allergan for 10–15 years. Doctors who improved their professional level with the company confidently stated that the constant development of their skills allows them to keep up with the times, with current trends, taking into account the dramatic changes that are now taking place in cosmetology and the industry as a whole.
“Currently, patients have begun to place more stringent demands on cosmetic procedures, their effectiveness, safety, longevity and timing of post-procedural rehabilitation,” noted Ekaterina Gutop , dermatovenerologist, cosmetologist, candidate of medical sciences, teacher at the Department of Dermatovenereology and Cosmetology Academy of the Federal Scientific and Clinical Center of the Federal Medical and Biological Agency of Russia and lecturer at MGIDO, - the main trends of anti-aging programs are natural results and the impression of a well-groomed, positive and fresh face; For young patients, there is a pressing request for correction that emphasizes gender accents – cheekbones and lips, and creates the effect of radiant skin.”
These trends are forcing cosmetologists to change their approaches to work.
“Highly values the long-term partnership with doctors. Only together we can constantly improve and raise the level of the aesthetic medicine industry in Russia! We will continue to develop our training programs, and this is only part of our path to innovation in the Russian market,” emphasized Ivan Melnik.
To confirm his words, Ivan Melnik announced a visit to Russia this year of the outstanding plastic surgeon from Brazil, Mauricio de Mayo, who is famous for developing a system of codes - points for introducing fillers into certain areas of the face for effective and noticeable lifting, as well as facial sculpting. Seminars with the participation of Mauricio de Mayo will become available to a wide range of Russian cosmetologists as part of training programs in Russia.
What should those who have installed Natrelle implants do?
Listen to your plastic surgeon's recommendations:
- Get examined 2 times a year: Ultrasound of the mammary glands.
- Visit a mammologist at least once a year.
- Do a breast self-examination once a month.
- Observe your body and the slightest changes in your breasts.
- Tell your doctor and get tested if you have any complaints.
Such recommendations are addressed not only to those patients who have had Allergan Natrelle installed, but to everyone who has undergone breast augmentation or is just planning surgery.
- Drinking through a straw, squinting... and 3 more crimes against the skin
- 30 of 884
- VI beauty award “Crystal Lotus”: the best plastic surgeon for mastopexy was named
“We are not afraid to raise stars.” What Allergan teaches cosmetologists
The results of the National Study of the Cosmetic Injections Market, conducted annually by the Vademecum Analytical Center, regularly point to the same circumstance - key decisions in this industry, including the choice of drugs and medical devices, are made by the doctor, and not by the clinic administration or the patient. For example, when asked who chooses a certain brand of botulinum toxin for injection, 65% of cosmetologists surveyed by Vademecum in 2022 answered that they do it, 34.4% referred to the clinic administration and less than 1% indicated that the choice of product depends on the patient. Almost identical indicators were recorded in the results of surveys about the features of choosing fillers and biorevitalizants for the corresponding procedures. Thus, it is the cosmetologist who influences patients’ adherence to certain drugs and, as a result, the demand and share of legitimate products in this industry. In this regard, the largest companies producing cosmetic injections, which also annually participate in the Vademecum study, note that one of the priorities of their work is training cosmetologists and improving their professional level.
In the summer of 2022, Allergan opened an updated innovative educational center, the Allergan Medical Institute, which features modular programs aimed at cosmetologists with different practical experience and levels of training.
The updated center is able to offer cosmetologists more than 100 educational master classes per year and train up to 2,500 specialists annually.
“The updated training center provides Russian doctors with various opportunities to master the world’s best practices and trends in the field of cosmetology,” notes Nina Nasledukhova, director of the business unit of aesthetic medicine at Allergan in Russia. The center's programs are focused not only on improving the skills of cosmetologists, but also on conveying to them the core values and philosophy of the company, aimed at improving the quality of life of people through innovative developments, quality products and scientific support for clinical practice.
Allergan’s first educational programs were launched back in 2012, and it was they that significantly helped the company to form a community of cosmetologists in Russia, whose priority was patient care and commitment to innovative products and techniques. Over seven years, the company has conducted more than 3 thousand master classes for specialists from different regions of Russia. Every year, more than 3 thousand doctors improve their professional level thanks to Allergan training events. The updated training center will allow the company’s educational direction to reach a fundamentally new level of development. “It is extremely important to use drugs correctly, since in inept hands they will not give the necessary and predictable results. Therefore, supporting educational programs and events for cosmetologists is one of the key priorities of our company,” notes General Director in Russia Mark Wilson.
Aesthetic clinics, having become familiar with the educational practices of experts, are also beginning to pay more attention to the training of their specialists. According to the general director of the Tori clinic, Olga Larina, the task of any doctor is to learn every day. And the specialists of her clinic have every opportunity to improve their professional qualifications. “Objectively speaking, there has always been a gray market and there probably always will be. Patients regularly ask our doctors to see them at home. And our doctors are always extremely indignant at such proposals and categorically answer: “No!” Because the doctors of our clinic have all the conditions to provide high-quality and safe services to the patient,” Olga Larina is convinced.
The problem of the “gray” segment has indeed not lost its relevance. According to the Vademecum Analytical Center, more than a quarter of this market is occupied by services provided at home, as well as with the use of unregistered drugs and medical devices. However, joint efforts by cosmetic injection providers and clinics to ensure that specialists are committed to quality products and legal service practices can radically change the situation in the industry.
Allergan-Natrelle breast implants (McGhan)
Unfortunately, we currently do not use Allergan implants, because... their permission to use has been revoked.
None of our patients (or the patients of our friends) encountered problems attributed to Allergan implants.
After many years, we will most likely find out whether there really was a problem or whether it was just a trade war, but the law is the law...
The Allergan campaign sent information letters, which are reproduced below without abbreviations:
“July 30, 2022 Dear Patient, In response to your request, on behalf of Allergan, we would like to inform you of the recent recall of BIOCELL® Textured Breast Implants and/or Tissue Expanders. These products were sold at various times under the trade names Inamed, McGhan and NATRELLE®. The company announced this voluntary recall and discontinuation of sales of all unused BIOCELL® textured breast implants and tissue expanders worldwide on July 24, 2022. The Federal Service for Surveillance in Healthcare was notified by Allergan CIS SARL LLC on July 26, 2022. This action is a precautionary measure due to recently updated global safety data obtained and published by the US Food and Drug Administration (FDA) regarding the uncommon occurrence of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). A voluntary recall means that Allergan has decided not to manufacture or sell these types of implants and to recall unused product in all markets where it operates. This recall does not mean that your implants should be removed or replaced unless you have specific symptoms. The recall only applies to BIOCELL® line products currently in healthcare settings. It is important that the US Food and Drug Administration (FDA) and other health regulatory authorities do not recommend prophylactic removal (explantation) or replacement of textured breast implants and other types of breast implants in patients without symptoms of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) due to the low risk of developing this disease. The main symptoms of BIA-ALCL are persistent swelling or pain in the breast implant area. These symptoms may appear well after the surgical incision has healed, usually years after the implant is placed. If you experience any of the above or other symptoms, please consult your healthcare provider. BIA-ALCL is not breast cancer - it is a type of non-Hodgkin's lymphoma (a cancer of the immune system). In most cases, BIA-ALCL is diagnosed in the scar tissue and serous fluid surrounding the implant, but in some cases, the disease can also affect surrounding tissue. Currently, the overall incidence of BIA-ALCL is considered low. In most patients, BIA-ALCL is successfully treated with surgical removal of the implant and the scar tissue around it. However, BIA-ALCL is a serious disease. Some patients may require chemotherapy and/or radiotherapy. BIA-ALCL is rarely fatal, especially when diagnosed early and treated promptly. Patients with Allergan BIOCELL® breast implants or tissue expanders should consider the following important recommendations: • If you do not have any symptoms, removal of any type of breast implant is not recommended due to the low risk of developing BIA-ALCL. However, if you have any questions, please contact your healthcare provider. • Remember that symptoms of BIA-ALCL include prolonged swelling or pain at the breast implant site. It is necessary to monitor for any changes in the area of the breast implants. • If you have any of the following symptoms or other changes, consult your healthcare provider about the need for further testing. The screening procedure for BIA-ALCL usually includes: a physical examination, visual examination, and/or analysis of the fluid or tissue around the breast implant. To confirm the diagnosis of BIA-ALCL, it is important to undergo testing, as this entails a change in the type of surgery that should be performed. • If the diagnosis of BIA-ALCL is confirmed, the patient must have the breast implant and surrounding scar capsule removed, which is a more extensive operation • As with any other type of implant, it is recommended to retain information about the manufacturer, unique part number and model name of the implant. You may have received this information from your surgeon on an identification card. If you need any of this information, contact your surgeon or obtain information about your surgery from the facility where it was performed. • Please note that most cases of BIA-ALCL occur many years after breast implant placement. Talk to your surgeon about your risk of developing BIA-ALCL. This voluntary worldwide recall does not apply to smooth breast implants and MICROCELL® (microtextured) breast implants and tissue expanders. NATRELLE® smooth implants and tissue expanders remain available to doctors and patients. Patient health and safety are Allergan's key priorities. If there are any concerns, patients are advised to contact their plastic surgeon to discuss the benefit-risk profile of breast implants. For additional information, please call 8-495-974-03-53 and email. You can also obtain additional information about BIOCELL textured breast implants and their recall on the company’s website www.Allergan.com, www.Allergan.ru. More detailed information about the recall is also available on the website of the Federal Service for Surveillance in Healthcare https://www.roszdravnadzor.ru/news/18218. RU-BRT-1950009"
July 30, 2022 Dear Healthcare Professional, Allergan is announcing a voluntary recall of BIOCELL® textured breast implants and tissue expanders. This action is a precautionary measure due to recently updated global safety data obtained and published by the US Food and Drug Administration (FDA) regarding the uncommon occurrence of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) BIA-ALCL is not breast cancer - it is a type of non-Hodgkin's lymphoma (a cancer of the immune system). In most cases, BIA-ALCL is diagnosed in the scar tissue and serous fluid surrounding the implant, but in some cases, the disease can also affect surrounding tissue. Currently, the overall incidence of BIA-ALCL is considered low. In most patients, BIA-ALCL is successfully treated with surgical removal of the implant and the scar tissue around it. However, BIA-ALCL is a serious disease. Some patients may require chemotherapy and/or radiotherapy. BIA-ALCL is rarely fatal, especially when diagnosed early and treated promptly. It is important that the US Food and Drug Administration (FDA) and other health regulatory authorities do not recommend prophylactic removal (explantation) or replacement of textured breast implants and other types of breast implants in patients with asymptomatic anaplastic large cell lymphoma. associated with breast implants (BIA-ALCL) due to the low risk of developing this disease. The US Food and Drug Administration (FDA) published a press release and materials about the voluntary recall on its website. These materials include a section titled “Advice for Healthcare Professionals,” as well as a section for patients entitled “Important Advice for Patients If You Have BIOCELL® Breast Implants.” Please review these materials at: https://www.fda.gov/medical-devices/safety-communications/fda-takes-action-protect-patients-risk-certain-textured-breast-implants-requests-allergan This voluntary worldwide recall does not apply to smooth breast implants and MICROCELL® (microtextured) breast implants and tissue expanders. NATRELLE® smooth implants and tissue expanders remain available to doctors and patients. The recall was initiated in relation to the following products: 1. Registration certificate: FSZ 2009/05895 Breast implant, filled with gel, Natrelle options: • 410 LL, LM, LF, LX, ML, MM, MF, MX, FL, FM, FF, FX • ST 410 LL, LM, LF, LX, ML, MM, MF, MX, FL, FM, FF, FX • INSPIRA TRL, TRLP, TRM, TRF, TRX, TSL, TSLP, TSM, TSF, TSX • 510 LX, MX, FX 2. Registration certificate: FSZ 2010/06196 Tissue expander Natrelle 133 Plus design options: • Tissue expander Natrelle 133 Plus RU-BRT-1950012 Natrelle 133P-FV, Natrelle 133P-MV, Natrelle 133P-LV, Natrelle 133P -MX, Natrelle 133P-SX, Natrelle 133P-SV, Natrelle 133P-FX • Natrelle 133 Plus tissue expander with suture loops Natrelle T-133P-FV, Natrelle T-133P-MV, Natrelle T-133P-LV, Natrelle T- 133P-MX, Natrelle T-133P-SV, Natrelle T-133P-FX 3. Registration certificate: Federal Federal Law 2010/06197 Breast implant, filled with gel, additionally filled with saline solution, with injection port, Natrelle 150; Options: FH, SH What Next for Healthcare Professionals • Healthcare professionals should no longer use BIOCELL® Textured Breast Implants and Tissue Expanders and any unused products should be returned to Allergan. • Clients/Healthcare Professionals are advised to check for availability of BIOCELL® Tissue Expanders and immediately move them to the quarantine area. • Healthcare professionals are encouraged to discuss this review with patients and answer their questions. • If patients have questions that you find difficult to answer, these requests can be addressed to Allergan CIS SARL LLC by phone 8-495-974-03-53 and email address [email protected] • Information about the recall process will be provided to healthcare professionals by Allergan additionally. Possible questions from patients Below you will find a list of questions and answers for further discussion with patients: 1) Should patients with Allergan breast implants take steps to remove them? RU-BRT-1950012 Currently, there are no recommendations from any health authorities, including the US FDA, for the removal or prophylactic replacement of textured breast implants or tissue expanders for asymptomatic patients. Since all surgical procedures involve risks, patients are advised to discuss the risk-benefit ratio of surgery with their doctor. 2) What is BIA-ALCL? BIA-ALCL is not breast cancer - it is a type of non-Hodgkin's lymphoma (a cancer of the immune system). In most cases, BIA-ALCL is diagnosed in the scar tissue and serous fluid surrounding the implant, but in some cases, the disease can also affect surrounding tissue. Currently, the overall incidence of BIA-ALCL is considered low. In most patients, BIA-ALCL is successfully treated with surgical removal of the implant and the scar tissue around it. BIA-ALCL is rarely fatal, especially if diagnosed early and treated promptly. However, BIA-ALCL is a serious condition. Some patients may require chemotherapy and/or radiotherapy. 3) What is the cause of BIA-ALCL? The cause of BIA-ALCL is not yet clear. A multifactorial etiology of the disease is assumed. Risk factors include biofilm formation, chronic inflammation, genetic predisposition, and implant surface characteristics. Bacterial contamination is possible during surgery. The large surface area of a textured implant may increase the risk of bacterial contamination and biofilm formation, which in turn may lead to chronic inflammation.[1] [1] Deva AK, Adams WP Jr, Vickery K. The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg. 2013;132:1319–1328. RU-BRT-1950012 4) Will Allergan reimburse costs associated with the removal of textured breast implants? Allergan has a warranty program for patients receiving the company's breast implants that provides support to all women if the events described in the terms of the warranty program occur, including the provision of free replacement products and financial support for medical expenses not covered by medical the patient's insurance policy. The current warranty program does not cover preventative removal of products. Additionally, the US FDA and other health authorities do not recommend removal or replacement of textured breast implants in asymptomatic patients. 5) Why was this product not recalled or withdrawn by Allergan sooner? These actions follow the US FDA's latest update to global product safety information regarding the uncommon breast implant-associated complication of anaplastic large cell lymphoma (BIA-ALCL). The company received updated safety information from the US Food and Drug Administration (FDA). You can review the press release posted on the FDA website for this information. Allergan has requested data from the FDA to support the updated safety information. Patient health and safety are Allergan's key priorities. If any questions arise, patients are advised to contact their plastic surgeon to discuss the RU-BRT-1950012 benefit-risk profile of breast implants. For additional information on this issue, please contact Allergan CIS SARL LLC by phone 8-495-974-03-53 and email
Natrelle breast enlargement implants from Allergan (USA) offer a wide range of options and high quality workmanship.
For over 30 years, ALLERGAN (formerly INAMED and McGhan Medical Corporation) has set the standard for the breast implant industry. ALLERGAN is the undisputed leader in evidence-based aesthetic technologies in the field of breast plastic surgery. Allergan's Natrelle breast implants meet global quality control standards.
Natrelle implants from the ALLERGAN company are manufactured under strict quality control. All Natrelle implants, both round and anatomical in shape, have a special INTRASHIEL barrier layer that prevents silicone gel from leaking through the shell.
The barrier layer made using INTRASHIEL technology covers the implant on absolutely all sides, 3600 (including the patch), delays the diffusion of silicone, reduces permeability and reduces the likelihood of implant rupture.
The unique BIOCELL texture is also used, which prevents implant displacement and promotes tissue fusion, which helps minimize the risk of complications such as implant rotation and capsular contracture.
The BIOCELL texture ensures the stability of the implant in relation to the soft tissues of the breast, effectiveness in reducing complications in the form of capsular contractures, a low percentage of capsular contractures and rotation, including anatomical implants.
Finished implants undergo more than a hundred stages of quality control; materials are also evaluated and tested. All types of implants come with a lifetime warranty.
Allergan is the leader in evidence-based aesthetic technologies in breast plastic surgery. Allergan Natrelle implants provide complete satisfaction to women who have had breast enlargement using these implants. Thanks to Natrelle implants, you will become the owner of a natural and proportional breast with long-term preservation of the result and projection.
The Allergan campaign offers unique solutions through a wide selection of NATRELLE gel-filled endoprostheses of high quality and reliability. A sophisticated selection of implants from a wide collection of gel-filled endoprostheses style 410, style 510 and INSPIRA provide excellent individual results and high levels of patient satisfaction.
Natrelle implants from the company ALLERGAN (Allergan) are a huge line of shapes, sizes and degrees of cohesiveness (elasticity).
INSPIRA (Round implants, 142 options) Possibility of an individual solution.
Optimal gel filling with minimal wrinkling
- Long lasting results
- The ideal solution to asymmetry
- 4 profile options;
- 2 options for gel filling: silicone gel, cohesive gel of extreme softness Soft touch.
Style 410 (Anatomical, teardrop implants, 119 options) Natural perfection of form.
- Repeats the natural shape and proportions of the breast.
- Low rating for capsular contractures.
- An ideal solution for correcting asymmetry.
- Wide range of options for customized solutions
- 12 anatomical shapes;
- 3 height options;
- 4 profile options;
- 2 options for gel filling: cohesive gel of extreme softness Soft touch, cohesive gel.
Style 510 (Anatomical implants, double gel, 39 options). Great solution.
- For patients with thin covering tissues
- For delicate breast shape correction without additional scars.
- For tubular breasts.
- In cases of reconstructive surgery.
Confidence in the result
- Impalpability of the implant due to the smoothness of the edges.
- Concave base ensuring maximum compliance of the implant shape with the tissues.
- The standard cohesive gel is located only at the base of the endoprosthesis. To ensure sufficient projection, a dense cohesive gel is placed in the anterior apical part of the implant.
- 3 height options;
- unique filling with two types of shape memory gel: cohesive gel, dense cohesive gel.
Each woman needs an individual approach when choosing breast implants.
All types of implants come with a lifetime warranty.
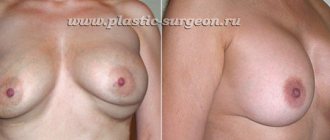
Photos before and after breast augmentation.
Sincerely, your plastic surgeon Zelenkova N.V.