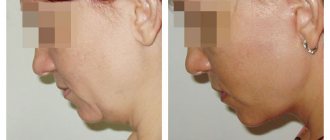
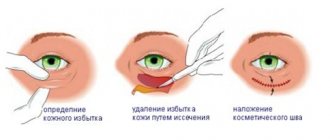
How will the new law on plastic surgery, which came into force on May 31, 2018, affect the activities of clinics? What will change for patients and medical staff, according to Order No. 298n? What will need to be changed in the internal structure and comprehensive equipment of the offices?
First, it is worth noting that the Order “On approval of the Procedure for providing medical care in the field of plastic surgery” replaced the old document No. 555n of the Ministry of Health of the Russian Federation dated October 30, 2012 due to the fact that mortality from such operations remains at a fairly high level. Order No. 298n dated May 31, 2018 is intended to protect clients of aesthetic medicine and plastic surgery centers. It is planned that the quality of services will improve significantly with the latest introductions.
Features and changes
The regulations apply to individual offices, blocks and entire plastic surgery centers. Now complex equipment is more strictly regulated, which should oblige the owners of medical centers to comply with certain standards and purchase everything necessary to undergo the licensing procedure.
Activities for the purchase of equipment and medical equipment intended for examinations, surgical operations and maintaining the condition of patients during the rehabilitation period will be strictly controlled. Those clinics that do not introduce the required changes in their work, according to Order No. 298n dated May 31, 2018, will be forced to cease operations.
What points does the new law correct?
The document touches on the most important aspects of the activities of plastic surgery clinics, from the equipment of offices, departments and complex centers to staffing standards and the organization of work of medical personnel. Based on the changes contained in the new law on plastic surgery, MEDMART LLC has streamlined the section of Ready-made solutions for clinics of this career guidance.
Order No. 298n dated May 31, 2022 approves the procedure for the provision of services. Types of medical care that a modern plastic surgery center should provide:
- urgent, emergency and planned;
- primary specialized and specialized. In this case, health care involves services involving high-tech equipment and technology.
The new law also regulates inpatient and outpatient conditions. The conditions in which assistance should be provided to patients under round-the-clock supervision or staying in a day hospital have been studied in more detail.
The activities of the surgeon, who is allowed to perform surgical interventions, are also regulated. A certified specialist must provide services only in an office that fully complies with this order. The presence of a plastic surgeon on the hospital staff does not mean the ability to conduct full-fledged activities. Operations in the field of “plastic surgery” can and should be carried out exclusively in those premises/offices that comply with the new law in absolutely all respects.
Plastic surgery and new requirements for clinics in 2022
21.08.2018
New rules of plastic surgery. What changes were made by order of the Ministry of Health No. 298n dated May 31, 2018?
Order of the Ministry of Health of the Russian Federation dated May 31, 2022 No. 298n “On approval of the Procedure for the provision of medical care in the field of plastic surgery,” which entered into force on July 3, 2022, made many changes to the activities of most plastic surgery clinics; it was adopted due to the increasing frequency of cases of death of plastic surgeons' patients as a result of the provision of poor-quality services.
Due to new requirements, many clinics and surgeons will no longer be able to provide services in this area.
The new law regulates the organization of activities, equipment standards and staffing standards:
- plastic surgeon's office;
- departments of plastic surgery;
- plastic surgery center.
The old order No. 555n of the Ministry of Health of the Russian Federation dated October 30, 2012 “On approval of the Procedure for providing medical care in the field of plastic surgery” with the release of the new law was declared invalid.
Who can perform plastic surgery?
According to the old order, specialized medical care could be provided by plastic surgeons and “surgical specialists who have undergone thematic advanced training in the relevant section of plastic surgery.”
Now this has become impossible.
A specialist who meets the qualification requirements for medical and pharmaceutical workers with higher education in the field of training “Healthcare and Medical Sciences” in the specialty “plastic surgery” is appointed to the position of a plastic surgeon in the office.
In accordance with the new law, plastic surgery departments and centers must provide advisory assistance to medical specialists in the following areas:
- therapy
- neurology
- dermatovenerology
- pediatrics
- otorhinolaryngology
- ophthalmology
- obstetrics and gynecology
- surgery
- urology
- Maxillofacial Surgery
- traumatology and orthopedics
If there are no necessary doctors in a medical organization, it is allowed to attract specialists from other medical organizations under a contract, provided that such medical organizations have a license for the relevant types of work.
Difficulties in correcting violations
The order was adopted unexpectedly; there was no time to prepare for the new requirements; immediately after the order came into force, inspections began, and they were followed by the mass closure of clinics.
During these events, many specialists lost their jobs, even if there were no violations or sad outcomes in their practice.
An experienced lawyer will help you understand and resolve the situation for clinics and practicing surgeons. If you treat this situation responsibly, you will be able to get out of it, and perhaps in more than one way.
Indications for plastic surgery – a fresh look
What has changed dramatically is the influence of the wishes of the clinic patients themselves. Now ambitions about one’s own appearance and demands for its correction are not the primary impetus for the surgeon’s actions. The new law, which came into force on May 31, 2018 in Russia, limits specialists’ activities if there are no compelling medical indications for carrying out a particular intervention.
All changes compared to the previous document are described in detail in paragraph 2 of the law. Maintaining and restoring human health became a priority. The purpose of the services that aesthetic medicine centers should offer is to return the patient to normal life after an accident, injury, complex illness and the use of potent drugs. The activities of the clinics can be briefly described as follows:
- elimination of functional defects;
- return of primary anatomical data lost for various reasons;
- correction of iatrogenic abnormalities, genetic, congenital or acquired during life;
- adjustment of appearance, as close as possible to generally accepted ideas about a person’s appearance;
- traumatic amputation of various limbs for medical reasons;
- reconstructive plastic surgery, which makes it possible to build up bones and soft parts in places of damage;
- fight against age-related changes. Here it is permissible to touch different parts of the head and body of a person seeking professional help;
- changes in the anatomy of individual structures, underlying and integumentary tissues, which helps the patient return to his usual way of life.
If the desire of any person who turned to a certified specialist was previously placed at the forefront, today such wording has been completely removed from the regulatory document. It will no longer be possible to change your appearance without any need or obvious reasons. The doctor himself will determine the need for a particular operation, based on the positive aspects that his patient will gain after a particular event or procedure.
Plastic surgery will come as close as possible to traditional medicine, aimed at improving and maintaining health and prolonging the normal life of citizens. Changing the shape of your nose or ears, succumbing to fashion trends or your own mood, will not work!
It is the transition of plastic surgery and the aesthetic medicine sector into a purely medical direction that is the most valuable change that should occur with the introduction of a new law into practice in Russia.
Order No. 298n dated May 31, 2018 and a new plastic surgeon
Who will the new doctors be, how will they work? The outdated Order was imperfect in many ways. Experts believe that his main mistake was allowing complex operations that could lead to fatal consequences for people’s lives to be carried out by surgeons who had undergone short-term training in performing certain surgical interventions.
With the innovations, the circle of those doctors, even long-term practicing surgeons, who will be allowed to perform specific surgical interventions will sharply narrow. The competence of plastic surgeons remains the entire range of measures to change the anatomical and structural changes in different parts of the patient’s body. And surgeons who specialized in other areas, but have completed courses to change their qualifications, will have to return to conducting activities in their main profile. Full training lasts for several years, and the skills acquired in practice provide the basis for error-free operation.
Thus, the new law with all its amendments will help remove from the segment of providing services in plastic and aesthetic surgery centers those doctors who do not have sufficient competence in this matter.
Given the transition from the patient’s desire to improve his appearance to every possible contribution to maintaining the health of citizens, centers should provide consultations on a variety of areas of medicine. This means that the staff must be increased. And clients of plastic surgery centers will now be able to receive information before any operation from:
- pediatrician and neonatologist (for pediatric surgery), therapist;
- ENT doctor;
- gynecologist;
- neurologist and neuropathologist;
- urologist;
- orthopedist-traumatologist;
- ophthalmologist;
- cosmetologist and dermatovenerologist;
- a surgeon involved in the restoration of the maxillofacial apparatus.
This approach is designed to eliminate mistakes that were made in practice earlier. Patients will be informed in advance about the quality, volume, and types of activities carried out. They will receive advice on possible changes in the functioning of various organs and life support systems, both negative and positive. Every citizen who decides to undergo plastic surgery of his own free will or for medical reasons will be familiar with the possible risks and the consequences arising from them. This is precisely what should reduce the flow of patients who experiment with their appearance and decide to undergo anatomical changes without good reason.
How will the work of clinics change?
Not everything is as confusing and complicated as it seems at first glance! The new law and Order on plastic surgery does not force centers to significantly increase the costs of training and hiring specialized specialists in various fields. If there is no doctor on the staff, he can be hired to work under a part-time contract. The main thing here is for patients to receive information about the state of their own health, its possible improvement or deterioration as a result of the provision of certain services within the plastic surgery center.
Important points when comprehensively equipping offices
Staff standards do not apply to private clinics. The equipment of the offices is described in Appendix No. 1, which indicates that now such premises should be included in the centers of medical complexes. Surgeons who do not meet the qualification requirements for the plastic surgery profile will not be allowed to practice.
The second appendix to the Order dated May 31, 2018 regulates the joint activities of surgeons and nurses, of whom there must be at least one per doctor. At least 1 sanitary worker must be allocated for every 3 premises. Appendix No. 6 is devoted to the preparation of the plastic surgery department for licensing. At the same time, the operating unit has its own amendments to the list of equipment.
The office should be clearly zoned - a dressing room and a place for conducting primary and routine examinations with all the necessary equipment. Invasive activities should not be carried out in the general office. Only dressings are allowed after operations when the patient’s condition is stable. Among the types of anesthesia, only application anesthesia is allowed; other types of anesthesia are carried out in the office of a plastic surgeon.
The third appendix of the new Order is devoted to the correct equipment standards. It contains tables with a list of mandatory and additional (recommended) equipment, consumables, etc.
Subsequent applications regulate the work of plastic surgery centers and departments within multidisciplinary hospitals. All premises of the department in which plastic surgery operations are performed must be located in one building or part of a building. When moving a patient, the patient must not be taken out/out of one building; it is allowed to move from building to building along warm, equipped passages.
New rooms in the plastic surgery center:
- X-ray room with a modern apparatus. A tomograph or x-ray is optional only for dental clinics. In general clinical centers where a variety of operations are performed, MRI and mammographs should be installed to examine the mammary glands;
- anesthesiology room/department.;
- resuscitation with PIT for the necessary equipment depending on the focus - children's or adult center;
- laboratory for diagnostic and clinical studies;
- dressing room;
- operating unit;
- an office or department of transfusiology with blood transfusion machines.
Order No. 298n, comments by Alexander Nerobeev
Around the Order of the Ministry of Health of the Russian Federation dated May 31, 2018 No. 298n “On approval of the Procedure for providing medical care in the field of plastic surgery”
Today there is a lot of talk, we could not ignore this topic and asked questions that concern many specialists and patients to the famous surgeon, member of the commission of the Ministry of Health of the Russian Federation on this issue -
Alexander Nerobeev
.
Alexander Ivanovich, how do you assess Order No. 298n?
I positively assess the adopted order.
Its main goal is to ensure that clinics providing plastic surgery services have the necessary equipment and qualified personnel. I admit that the amount of equipment that a clinic should be equipped with is to some extent excessive; an example is a computed tomograph. Its cost reaches more than $1 million, and for obvious reasons small clinics cannot afford it. But not everyone should undergo plastic surgery!
I recently returned from a business trip to Mongolia. There I visited one of the clinics. It is privately owned, but at the same time it is equipped with equipment that can only be found here at the Institute of Neurosurgery. The clinic has a CT scanner and even a blood transfusion room. In fact, you can perform any operation in it - from rhinoplasty to complex manipulations on the intestines. I think this approach is correct. Patients should not die because they cannot receive qualified care on site.
Another problem of modern clinics is the lack of permits for this medical activity. Based on the results of an inspection of 150 clinics in the North Caucasus, it was revealed that only 10 of them have the necessary documents. The rest of the clinics simply take advantage of the low literacy of the local population in the provision of medical services.
Also in our country there is an acute issue with education in the field of plastic surgery.
World practice shows that in order to become a plastic surgeon, you need to study for at least 10 years, including your first higher medical education. As a rule, plastic surgery involves specialists with experience in surgery who are able to provide assistance in critical situations. In Russia, it has developed so that, having received an education at a medical institute, yesterday's students study for 2 years in residency and after that they immediately begin to operate. I, as the head of the Department of Plastic Surgery at the Russian Medical Academy of Postgraduate Education, can say that a group of 15 people is a lot. In such conditions, it is not possible to take all the residents to operations each time and individually analyze the topics. I could work more effectively with groups of 2-3 residents, but, unfortunately, this is not yet possible.
It is also worth noting that many residents come to plastic surgery for extra income. They are only interested in easy operations that will bring them money, but they are not at all ready, for example, to spend 1.5-2 hours to remove a small scar. Such operations, of course, do not bring that same super income.
The level of first higher education also leaves much to be desired. Sometimes we come across very “weak” students who want to operate. Some plan to work only with fillers. I do not agree with this approach to the profession.
I also categorically disagree with the fact that many plastic surgeons see people not as patients, but as clients. They are not ready to dissuade you from unnecessary operations; they often impose money in this way.
Do you have information about how many clinics were closed in Moscow due to non-compliance with the requirements of Order No. 298n?
There is no exact information. Some clinics receive a six-month deferment with the condition that all necessary equipment will be purchased.
What are the requirements for equipping clinics abroad?
Different countries have different requirements for the necessary equipment, and indeed for the performance of operations and the experience of plastic surgeons. In the USA, for example, “office surgery” is common. I visited such an “office”. It is located in an 18-story bank building. The clinic rents the third floor. There is no one-day hospital. The patient is operated on and sent home. In the event of an incident, numerous lawyers will be involved on behalf of the patient, and the clinic, as well as the operating doctor, will be held liable. Plus, in the USA, both the doctor and the patient must be insured by an insurance company.
If we take Europe as an example, then in Germany, for example, there used to be a rule that prohibited a doctor from working in the field of aesthetic medicine until he reached the age of 35. Advertising for plastic surgery is virtually banned in France.
With us it’s the other way around. Many surgeons, without even having experience, write in advertising articles that they are among the three best surgeons in Russia. In this case, the person may not be even 30 years old. It is not clear how such success could be achieved by this age, and where the competition took place, according to the results of which such a surgeon entered the top three... We need to strictly regulate work in the field of plastic surgery in order to cut off those who perform their work poorly and dishonestly.
Does ART Clinic meet the requirements of Order No. 298n?
The clinic has existed for 15 years. It is integrated into the Institute of Neurosurgery named after. Burdenko. Any patient coming to our clinic has access to all services provided by other departments. In the event of an emergency, the patient will be provided with comprehensive, qualified assistance. The clinic staff includes 8 plastic surgeons who specialize in maxillofacial, general and plastic surgery. Most of them are my students. Surgeons have an advanced degree and have trained for a total of at least 11 to 12 years.
I also want to add about education.
The issue of increasing the mandatory minimum of additional education from 2 years to 4 is currently being considered. Work has been suspended for now, as the financial side of the issue needs to be resolved. Currently, residents receive a stipend of about 7,500 rubles. Naturally, it is impossible to live on these funds, especially since many people start families by the age of 30. A person has to earn extra money, which means he cannot study fully. Abroad, interns (residents) have the opportunity to enter into an agreement with a clinic that will provide housing for the period of study, which is from 2 to 4 years, as well as cash - 1,500 - 2,000 dollars per month. According to the terms of the contract, a person is obliged to work in the clinic that provided him with residency. Every six months of training, residents gain experience in various areas: surgery, anesthesiology, etc. Based on the results of the training, the clinic receives a specialist with experience, which is beneficial for both parties. In Russia, we have yet to develop an effective scheme for supporting specialists so that they can safely complete their studies and begin operating.
What professional qualities does a plastic surgeon need to have to work in your clinic?
The clinic is currently staffed. If we consider a new specialist, then, first of all, he must be a professional. Negotiations with doctors are conducted individually. Perhaps the specialist knows how to perform operations that would be of interest to our clinic, but we don’t do them yet; of course, we will consider the candidacy. We do not give preference to our students. It is important for us that the surgeon can work in accordance with the style of the clinic - does not impose unnecessary things and respects the patient and his needs.
Do I need to be a qualified maxillofacial surgeon to perform facial surgery?
About 7 years ago, all facial surgeries were classified as dentistry, and most plastic surgeons came from dentistry. Nowadays, plastic surgery includes not only operations on the face: they perform operations on the mammary glands, perform plastic surgery on the body, etc. Therefore, there is now a clear division between dentistry and maxillofacial surgery. A dental surgeon performs operations only in the oral cavity. A maxillofacial specialist can perform facial surgery, but he must undergo training in plastic surgery.
What operations do you personally carry out?
Due to the fact that I run a large clinic, I now only undertake complex operations. We have good specialists who I can completely trust to carry out operations and carry out management work.
How to choose the right plastic surgery clinic?
You need to collect all possible information about the clinic. Check how long the clinic has been operating and how it is equipped. Pay attention to the age and education of doctors, what publications they make, what achievements they have. The location of the clinic in the city is also of great importance. Of course, you should not opt for clinics located in basements and with a minimal set of equipment and tools. In this case, cheap does not mean good.
Alexander Ivanovich, thank you for the interesting conversation!
Conclusions on the implementation of the new law
If previously there was no office for transfusiology and blood transfusion, now it is mandatory. A 24-hour hospital is being introduced, in addition to the operating day hospital and outpatient clinic. The room with X-ray equipment is equipped in strict accordance with the list. You can forget about redirecting patients to third-party centers for such studies.
Surgeons who have undergone advanced training will not be involved in performing plastic surgeries. All serious manipulations can only be performed by specialized specialists who have undergone full training and received a diploma with qualifications in the relevant profile.
List of laws, orders and SanPinov
LIST OF LAWS, ORDERS AND SanPinov:
1. Constitution of the Russian Federation, Article 41
“The right to health protection and medical care.”
2. Federal Law No. 323 dated November 21, 2011
“On the basics of protecting the health of citizens in the Russian Federation.”
3. Law on medical insurance of citizens of the Russian Federation No. 1499-1 of June 22, 2006.
4. Federal Law No. 61 dated April 12, 2010
“On the circulation of medicines.”
5. Federal Law No. 77 dated June 18, 2001
(edited on July 18, 2011) “On preventing the spread of tuberculosis.”
6. Federal Law No. 52 of March 30, 1999
(as amended on November 25, 2013) “On the sanitary and epidemiological welfare of the population.”
7. Federal Law of the Russian Federation No. 157 of September 17, 1998
“On immunoprophylaxis of infectious diseases.”
8. Federal Law No. 3 of 01/08/1998
“On narcotic drugs and psychotropic substances.”
9. Federal Law of the Russian Federation No. 38 of March 30, 1995.
“On the prevention and spread in the Russian Federation of the disease caused by the human immunodeficiency virus (HIV infection).”
10. Federal Law of 01/09/1996
N 3-FZ
(as amended on 07/19/2011) “On Radiation Safety of the Population”.
11. Order of the Ministry of Health of the RSFSR dated 02.08.1991 N 132
(as amended on 04/05/1996) “On improving the radiology diagnostic service.” Appendix No. 9. Regulations on the radiologist of the X-ray department (office) of the department (department) of radiology diagnostics.
12. Order of the Ministry of Health of Russia dated November 15, 2012 N 932n
“On approval of the Procedure for providing medical care to patients with tuberculosis” (Registered with the Ministry of Justice of Russia on 03/07/2013 N 27557)
13. Letter of Rospotrebnadzor dated April 21, 2010 N 01/6161-10-32
“On the procedure for admitting new medical technologies to medical use” (together with the Letter of Roszdravnadzor dated March 23, 2010 N 03-6315/10 “On the procedure for using methods of radiation diagnostics and therapy”).
14. Order of the Ministry of Health of the Russian Federation N 239, Gosatomnadzor of the Russian Federation N 66, State Committee for Ecology of the Russian Federation N 288 dated 06/21/1999
“On approval of guidelines” (together with the “Procedure for maintaining radiation and hygienic passports of organizations and territories (guidelines)”).
15. Order of the Ministry of Health of the Russian Federation dated January 28, 2002 N 19
“On the Standard Instructions on Occupational Safety and Health for X-ray Department Personnel” (Registered with the Ministry of Justice of the Russian Federation on April 17, 2002 N 3381).
16. Order of the Ministry of Health of the Russian Federation No. 125 of March 21, 2014.
“On approval of the national calendar of preventive vaccinations and the calendar of preventive vaccinations for epidemic indications.”
17. Order of the Ministry of Health of the Russian Federation No. 382n dated June 18, 2013.
“On the forms of medical documentation and statistical reporting used during medical examination of certain groups of the adult population and preventive medical examinations.”
18. Order of the Ministry of Health of the Russian Federation No. 378n dated June 17, 2013.
“On approval of the rules for registering operations related to the circulation of medicinal products for medical use, included in the list of medicinal products for medical use, subject to subject-quantitative accounting, in special journals for recording operations, narcotic drugs and psychotropic substances, registered in the prescribed manner in the Russian Federation in as medicines intended for medical use in pharmacies, medical institutions, research, educational organizations and wholesale trade organizations of medicines.”
19. Order of the Ministry of Health and Social Development of the Russian Federation No. 302n dated April 12, 2011.
“On approval of lists of harmful or dangerous production factors and work, during the performance of which mandatory preliminary and periodic medical examinations (examinations) are carried out, and the procedure for conducting mandatory preliminary and periodic medical examinations (examinations) of workers engaged in heavy work and in work with harmful or dangerous conditions labor."
20. Order of the Ministry of Health and Social Protection of the Russian Federation No. 706n dated August 23, 2010.
“On approval of rules for storing medicines.”
21. Order of the Ministry of Health and Social Protection of the Russian Federation No. 932n dated November 15, 2012.
“The procedure for providing medical care to patients with tuberculosis.”
22. Order of the Ministry of Health of the Russian Federation No. 1006 dated December 3, 2012.
“On approval of the procedure for medical examination of certain groups of the adult population.”
23. Order of the Ministry of Health of the Russian Federation No. 1011 dated December 6, 2012.
“On approval of the procedure for conducting preventive medical examination.”
24. Order of the Ministry of Health of the Russian Federation No. 1346 dated December 21, 2012.
“The procedure for minors to undergo medical examinations.”
25. Order of the Ministry of Health of the Russian Federation No. 342 dated January 26, 1998.
“On strengthening and improving measures for the prevention of typhus and pediculosis.”
26. Order No. 170 of 08.18.94.
“On measures to improve the prevention and treatment of HIV infection in the Russian Federation.”
27. Order of the Ministry of Health of the Russian Federation No. 36 dated 02/03/97.
“On improving measures for the prevention of diphtheria.”
28. Order No. 83 of 08/16/2004
“On the procedure for conducting preliminary and periodic medical examinations of workers, and medical regulations for admission to work in the profession.”
29. Order of the Ministry of Health of the Russian Federation No. 408 dated July 12, 1989.
“On measures to reduce the incidence of viral hepatitis.”
30. Order of the Ministry of Health of the Russian Federation No. 475 dated August 16, 1989.
“On measures to prevent acute intestinal infections.”
31. Order No. 02-08/10-1977P dated 08/21/2000.
“Approximate timing of VN for the most common diseases and injuries (in accordance with ICD-10).”
32. Order of the Ministry of Health of the Russian Federation dated April 23, 2013. No. 240n
“On the Procedure and timing for medical workers and pharmaceutical workers to undergo certification to obtain a qualification category.”
33. Methodical instructions.
Organization of differentiated fluorographic examination of the population in order to identify diseases of the thoracic cavity organs, approved.
Ministry of Health and Medical Industry of the Russian Federation, State Committee for Sanitary and Epidemiological Surveillance of the Russian Federation dated February 22, 1996 N 95/42.
34. SanPin 2.1.3.2630-10
“Sanitary and epidemiological requirements for organizations engaged in medical activities.”
35. SanPin 2.1.7.2790-10
“Sanitary and epidemiological requirements for the management of medical waste.”
36. OST 42-21-85
Industry standard, mandatory for implementation in all health care facilities, “Sterilization and disinfection of medical devices, methods, means, regimes.”
37. SanPin 3.1.5.2826-10
“Prevention of HIV infection” approved. 01/11/2011
38. SanPin 3.5.1378-03
“Sanitary and epidemiological requirements for the organization of disinfection activities.”
39. SanPin 3.1./3.2.3146-13
“General requirements for the prevention of infectious and parasitic diseases.”
40. SanPin 3.2.3110-13
"Prevention of enterobiasis."
41. SanPin 3.1.2.3109-13
"Prevention of diphtheria."
42. SanPin 3.1.1.3108-13
"Prevention of intestinal infections."
43. SanPin 3.1.2.3117-13
“Prevention of influenza and other acute respiratory viral infections.”
44. SanPin 3.1.2.3114-13
"Prevention of tuberculosis."
45. SanPin 3.1.2.2951-11
"Prevention of polio."
46. SanPin 3.1.2952-11
"Prevention of measles, rubella and mumps."
47. SanPin 3.1.7.2836-11
"Prevention of salmonellosis." Changes and additions No. 1 to SanPin 3.1.7.2616-10.
48. SanPin 3.1.7.2616-10
"Prevention of salmonellosis."
49. SanPin 2.4.4.2599-10
“Hygienic requirements for the design, maintenance and organization of the regime in health institutions with daytime stay for children during the holidays.”
50. SanPin 3.1.7.2615-10
"Prevention of yersiniosis."
51. SanPin 3.3.2.1248
“Conditions for transportation and storage of medical immunobiological preparations.”
52. SanPin 3.1.3112 from 2013
"Prevention of viral hepatitis C."
53. SanPin 3.1.2825 from 2010
"Prevention of hepatitis A."
54. SanPin 3.1.1.2341 from 2008
"Prevention of viral hepatitis B."
55. SanPin 3.1.2.3117 from 2013
"Prevention of ARVI."
56. SanPin 3.1.1.3108 from 2013
Prevention of acute intestinal infections."
57. SanPin 3.1.2.3109 from 2013
Prevention of diphtheria."
(0Mb)